About the report
The Health Foundation is an independent charity committed to bringing about better health and health care for people in the UK.
Health Data Research UK (HDR UK) is the UK’s national institute for health data science. HDR UK’s mission is to bring together the UK’s health and care data to enable discoveries that improve people’s lives by uniting, improving and using data as one national institute.
HDR UK’s Better Care programme aims to equip clinicians and patients in the UK with the best possible data-based information to make decisions about their care. Over the past 2 years, as part of this wider programme, the Health Foundation and HDR UK have been working in partnership to deliver the Better Care Catalyst programme. This has funded three projects to develop data-driven tools that aim to improve health care decision making and also supported three workstreams to set out the training, knowledge mobilisation and policy actions required to support data-driven learning and improvement in health care.
This report is the final output of the Better Care Catalyst programme’s policy and insights workstream, which researched the barriers and enablers for implementing learning health system approaches in the UK. It supports the wider Better Care programme and community by providing analysis and advice to further the use of data to improve health care services. It also identifies a range of opportunities and actions that policymakers and organisational and system leaders can take to advance the learning health systems agenda across the UK.
Key points and summary of recommendations
- A learning health system (LHS) is a team, provider or group of providers that, working with a community of stakeholders, has developed the ability to learn from the routine care it delivers and improve as a result – and, crucially, to do so as part of business as usual. Done right, LHSs are not a separate agenda, but about embedding improvement into the process of delivering health care.
- An LHS is a way of describing a systematic approach to iterative, data-driven improvement (regardless of whether those involved label it as an LHS). Learning and improvement are already happening in most providers and, in many cases, LHS approaches will offer a way to pull this existing work together in a more systematic way and organise it more effectively. In this sense, some see LHSs as the next stage in the evolution of traditional quality improvement approaches.
- Tackling the huge pressures that health and care services are currently under will require action on multiple fronts, notably recruiting more staff and increasing investment in services. Amid all these pressures, we should be wary about seeing LHSs as a ‘nice to have’. A step change in the health service’s learning and improvement capability is precisely what is required if it is to find a sustainable way out of the current crisis and effectively reshape care to meet future health needs.
- On the one hand, helping teams and providers become LHSs gives them the tools to diagnose and solve problems and to drive improvement from within – turning them into ‘engines of innovation and improvement’. Over the long term, this potentially offers a powerful and more sustainable route to improving quality and efficiency than simply relying on national programmes or external consultancy.
- On the other hand, LHSs can also be thought of as sophisticated ‘implementation mechanisms’, providing the infrastructure for teams and providers to effectively adapt, embed and refine ideas and innovations from elsewhere. By supporting teams and providers to adopt solutions and implement national change programmes effectively, LHSs can play an important role in helping to deliver national priorities for recovery and service transformation.
- While LHSs require technical capability to analyse data and implement improvements, they are also deeply social – requiring networks of people, collaboration and a conducive culture. Investing in getting this ‘human infrastructure’ right is just as important as the technical side. Ultimately, it is the ability of LHSs to bring people together to ask questions, interpret data, reconcile differing views and make decisions that allows them to successfully effect change in a complex, adaptive system such as health care.
- Our research – informed by a literature review, interviews, a survey of more than 100 expert stakeholders and a series of practical case studies – suggests there is a large gap between the promise and practice of LHSs. This is partly due to the lack of a clear definition, vision and evidence base around LHSs, meaning it can be difficult to know where to start or how to make progress.
- This report aims to demystify the concept of LHSs and explores four important areas especially relevant to LHSs where action can lead to tangible progress: learning from data, harnessing technology, nurturing learning communities and implementing improvements to services. Each of these areas is important in its own right, with much to be gained by making progress on each one individually. Indeed, for those wanting to create LHSs, the first step will often be to develop one or two components. But it is ultimately by bringing these components together into a full LHS that they can become more than the sum of their parts.
- To help facilitate LHS approaches and realise their benefits, policymakers and organisational and system leaders will need to make progress on a range of related policy agendas, such as data, digital maturity and interoperability, and improvement capability and culture. They will also need to develop a clear vision and narrative around LHSs. This report highlights eight key areas for action – summarised in Box 1 – to help overcome the challenges involved.
Box 1: Eight priority areas for action
This report highlights eight areas where action by policymakers (government ministers, civil servants and national leaders) and organisational and system leaders (those in leadership roles in providers and local and regional health care systems) could support the development of LHSs, as shown in Table 1. Further details can be found in Chapter 3.
Table 1: Eight priority areas for action and recommendations for each
|
Area for action |
Recommendations |
|
|
For policymakers |
1. Clear narrative |
|
|
2. Digital maturity |
|
|
|
3. Data analytical expertise |
|
|
|
4. System interoperability |
|
|
|
5. Implementation and improvement capability |
|
|
|
For organisational and system leaders |
6. Learning culture |
|
|
7. Front-line implementation capability |
|
|
|
8. Organisational improvement capability |
|
Introduction
The health and care system in the UK is facing some of the most significant challenges in its history. Services are finding themselves under huge pressure – a result of both the COVID-19 pandemic and a period of significant underinvestment. At the same time, the need to reshape care to better meet future health needs is becoming more urgent. Enabling services to recover and rising to meet these challenges will require the NHS to use every tool available.
Learning health systems (LHSs) offer one possible way to help services recover and improve, even within the current challenging circumstances. Rather than simply relying on ‘top-down’ national policy interventions alone, they harness the power of providers to drive improvement from within – and, moreover, to do so as part of ‘business as usual’. As such, LHSs can be powerful vehicles for improving services and population health. They may be particularly important for achieving successful service transformation over the next few years. For example, NHS England’s Transformation Directorate recently indicated that it sees LHS approaches as important for realising the benefits of new technologies in the NHS. The significant service innovations that were implemented rapidly after the onset of the COVID-19 pandemic show what can be achieved when people come together around a common ambition, when we maximise the use of data and when staff are supported to deliver change.
In many ways, health care has always been a form of ‘learning system’. But recent advances in data and technology, coupled with the move towards better collaboration and integration between services, are now presenting new opportunities to learn and improve in a more systematic way. Since the term ‘learning healthcare system’ was coined by the Institute of Medicine in the US in 2007, interest in the LHS agenda has been growing rapidly.
Despite this growing interest, most providers and systems have not yet been able to capitalise on the potential of LHSs. There are several reasons for this gap between promise and practice. There are many different conceptions of LHSs, as well as a lack of a robust, practical evidence base. This makes it difficult to forge consensus on what an LHS is and the benefits it can bring. There are a range of practical challenges with creating LHSs: the capabilities they require, such as data analytics and quality improvement skills, need nurturing in their own right, and attempts to do so often run up against wider structural, policy or resource barriers. At the same time, it can be hard for policymakers and organisational and system leaders to know how best to support this agenda.
The report
This report, part of Health Data Research UK’s (HDR UK’s) Better Care programme, aims to tackle these questions head-on. We seek to demystify LHSs and contribute to a clearer narrative and vision about the role LHS approaches can play in improving health and care. We also seek to identify the most pressing challenges, explore several important areas where targeted action by policymakers and organisational and system leaders could lead to tangible progress, and support policymakers and practitioners as they consider the next steps.
For this project we carried out desk research and conducted interviews with expert stakeholders. To further investigate the opportunities and challenges for LHSs, we conducted a purposive online survey of 109 expert stakeholders between December 2021 and January 2022, the results of which are described throughout this report. Our respondents represented a range of expertise from across the UK, both in LHSs and in the key areas of LHS activity, such as collecting and analysing data, engaging patients and the public, and quality improvement. We also explored existing examples of LHSs, with detailed investigation of 16 case studies. In addition, we drew together learning from the Health Foundation’s programmes and research across areas such as quality improvement, technology and data analytics, as well as our experience of supporting networks such as the Q community and the NHS-R community. We also drew on learning from HDR UK’s Better Care programme.
Content overview
Chapter 1
Chapter 1 looks at what LHSs are and the different types in existence, as well as highlighting the common activities and assets underpinning them all. It explores the growing interest in LHSs in the UK and considers why they might be particularly relevant over the coming years.
This chapter will be useful for those interested in the concept of LHSs and those who would like to understand how LHS approaches can address key health and care challenges and drive improvement.
Chapter 2
Chapter 2 considers four key areas that are particularly important for LHSs: learning from data, harnessing technology, nurturing learning communities and implementing improvements to services. For each, it details the key opportunities and challenges for LHSs, drawing on our survey evidence to identify which challenges are the most pressing.
This chapter will be useful for those wishing to understand in more detail the different aspects of LHSs and some current challenges for developing them. Some readers may already be familiar with the debates in particular sections (as many of the complexities facing LHSs are reflective of broader challenges) and so may wish to explore the sections with which they are less familiar.
Chapter 3
Chapter 3 explores key priorities for developing LHS approaches, as identified by our survey respondents. It also offers recommendations for how these priorities could be realised.
This chapter will be useful for policymakers and organisational and system leaders to understand what practical actions they can take to support the development of LHSs. It may also be useful for practitioners who wish to understand the broad spectrum of actions that can be taken to support LHS approaches.
Case studies
Throughout this report, we draw on 16 case studies to exemplify LHS approaches that are already being applied across the UK (see Box 2 for a summary).
Box 2: Case studies
We present 16 case studies in this report. As indicated in Table 2, some of the case studies are presented as examples of full LHSs, while others concentrate on one of the four areas discussed in Chapter 2.
Table 2: The 16 case studies and what they focus on
|
No |
Case study |
Used to illustrate… |
|
1 |
Flow Coaching Academy |
Full LHS |
|
2 |
PINCER – a pharmacist-led intervention to reduce medication errors |
Full LHS |
|
3 |
CFHealthHub – a digital learning health system |
Full LHS |
|
4 |
Nightingale bedside learning coordinator |
Full LHS |
|
5 |
The Clinical Effectiveness Group |
Full LHS |
|
6 |
The Children & Young People’s Health Partnership |
Full LHS |
|
7 |
The Secure Anonymised Information Linkage (SAIL) Databank |
Data |
|
8 |
Informatics Consult |
Data |
|
9 |
Towards a national learning health system for asthma in Scotland |
Data |
|
10 |
Reducing the health burden of diabetes with artificial intelligence-powered clinical decision tools (RADAR) |
Technology |
|
11 |
Cambridge University Hospitals’ eHospital programme |
Technology |
|
12 |
Project Breathe – artificial intelligence-driven clinical decision-making tools to manage cystic fibrosis |
Technology |
|
13 |
Thiscovery |
Learning community |
|
14 |
Q Lab UK |
Learning community |
|
15 |
HipQIP – hip fracture quality improvement programme |
Improvement |
|
16 |
Reducing brain injury through improving uptake of magnesium sulphate in preterm deliveries (PReCePT2) |
Improvement |
What are learning health systems and why do they matter?
1.1. What are learning health systems?
A learning health system (LHS) is a way of describing a team, provider or group of providers in the health and care system that, working with a community of stakeholders, has developed the ability to learn from its own delivery of routine care and improve as a result. At its most fundamental, an LHS comprises a set of activities and assets that enable continuous learning and improvement of services.
LHSs are an important method for improving the quality, efficiency and effectiveness of health and care services. But there are many different types of LHS, ranging from the clinical microsystem level to the national level and everything in between. As a result, the term tends to be used in many different ways. We think that this can, on occasion, be a stumbling block to making progress in this field – with people sometimes talking at cross purposes or using terminology in overly restrictive ways.
For that reason, we begin this chapter by developing an analytical framework for understanding the key components of LHSs and for characterising the variety of types that exist.
Common aspects of learning health systems
While there are many different types of LHS, they all have some key factors in common, although important variation can emerge in relation to these factors.
- The provision of services. At the core of an LHS sits a service provider or providers, and the desire to improve service provision and outcomes drives the LHS’s activity. An associated factor is that a key source of data from which an LHS learns is data generated from routine service provision (whether clinical data, operational data, patient-reported data and so on). This is one important thing that makes an LHS different from many types of research or trials relying purely on bespoke data collection. (Another important difference is the continuous, iterative nature of the learning that takes place within LHSs – discussed further below.) The presence of a provider means the type of improvement that LHSs do can be endogenous (driven from within) rather than simply exogenous (externally driven by factors such as policy or regulation).
One possible source of variation among LHSs is therefore the type and sector of the provider in question and the nature of services being delivered (for example, health care, public health, social care or community services). What is being improved will also affect who the ‘service users’ might be on any particular occasion (for example, patients, staff, carers or citizens).
- The learning community and improvement ambition. An LHS is driven by a learning community that has been formed around a common ambition of improving services and outcomes. Not everyone in the learning community will necessarily be involved in every stage of the LHS (for example, patients might be involved in formulating ideas and trialling service changes but not in data analysis; data analysts might be involved in generating learning from data but not implementing service changes; and so on). However, all share and contribute to the common ambition in some way.
Another critical source of LHS variation is therefore the nature of the learning community, and its corresponding improvement ambition:
- They could be place based, and if so they could exist over a range of geographies (for example, based around an individual provider organisation, a health economy or the whole NHS).
- They could be condition based (for example, improving care for people with cystic fibrosis).
- They could be thematic (for example, improving procurement, adopting a particular technology or reducing a particular type of medical error).
- They could combine these properties (for example, improving asthma care for young people in London).
- The learning and improvement cycle. LHSs effect change through iterative learning cycles based on generating and learning from data and formulating and testing service changes. Despite the huge diversity of LHSs, their learning and improvement cycles tend to be based on a common set of stages, illustrated in Figure 1. And at each stage of the cycle, the same types of activities tend to be going on: measuring outcomes, formulating hypotheses, analysing performance, designing improvements, implementing service changes and so on (see Figure 2).,, The cycle is then repeated, allowing each subsequent iteration to test and evaluate the service changes implemented in the previous iteration. These activities are the ‘bread and butter’ of LHSs. And it is by focusing on how to do these activities well, we can support the development of LHSs, whatever their form.
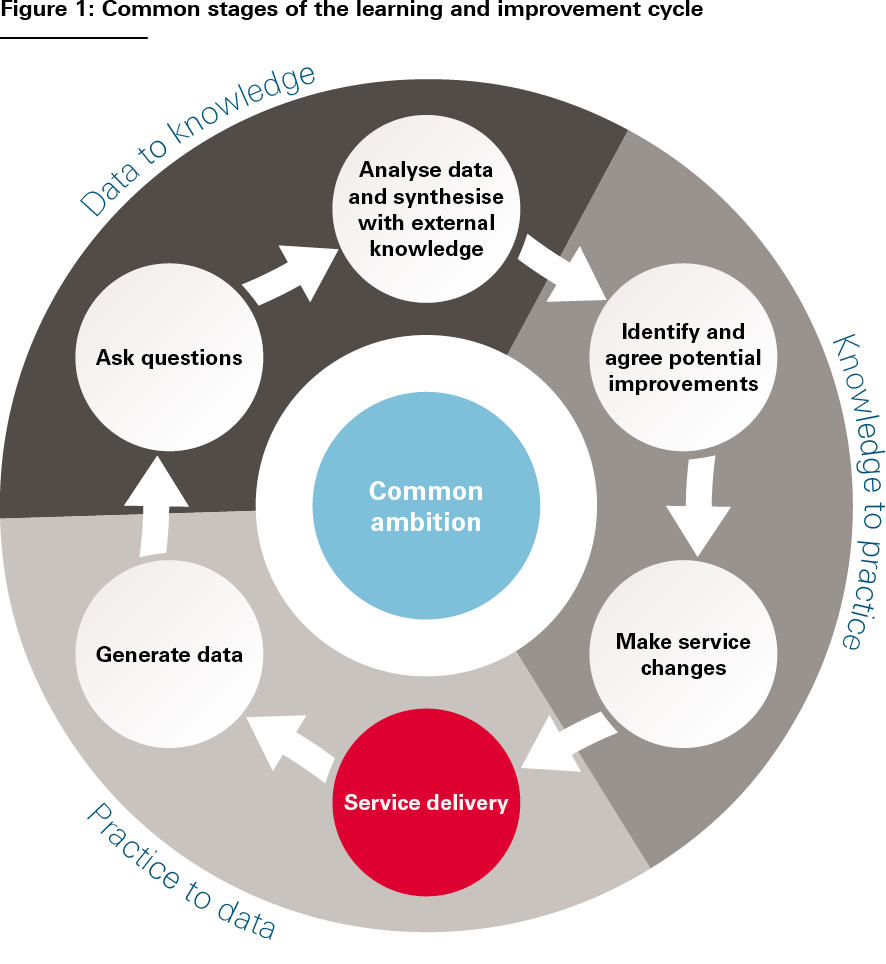
Source: The Health Foundation’s Insight & Analysis Unit
Importantly, if it is the presence of certain activities that constitutes an LHS, then it does not really matter whether the individuals involved think of it as an LHS or not. As we will see with some of the case studies explored in this report, something can be an LHS even if the practitioners involved do not use that terminology or did not set out explicitly to develop an LHS.
Figure 1 is not intended to be a systematic analysis of the learning and improvement cycle, but simply a useful way to think about the constituent activities that go on in an LHS. It broadly corresponds to Charles Friedman’s influential three-stage characterisation of the learning cycle: practice to data; data to knowledge; and knowledge to practice (indicated in the figure).
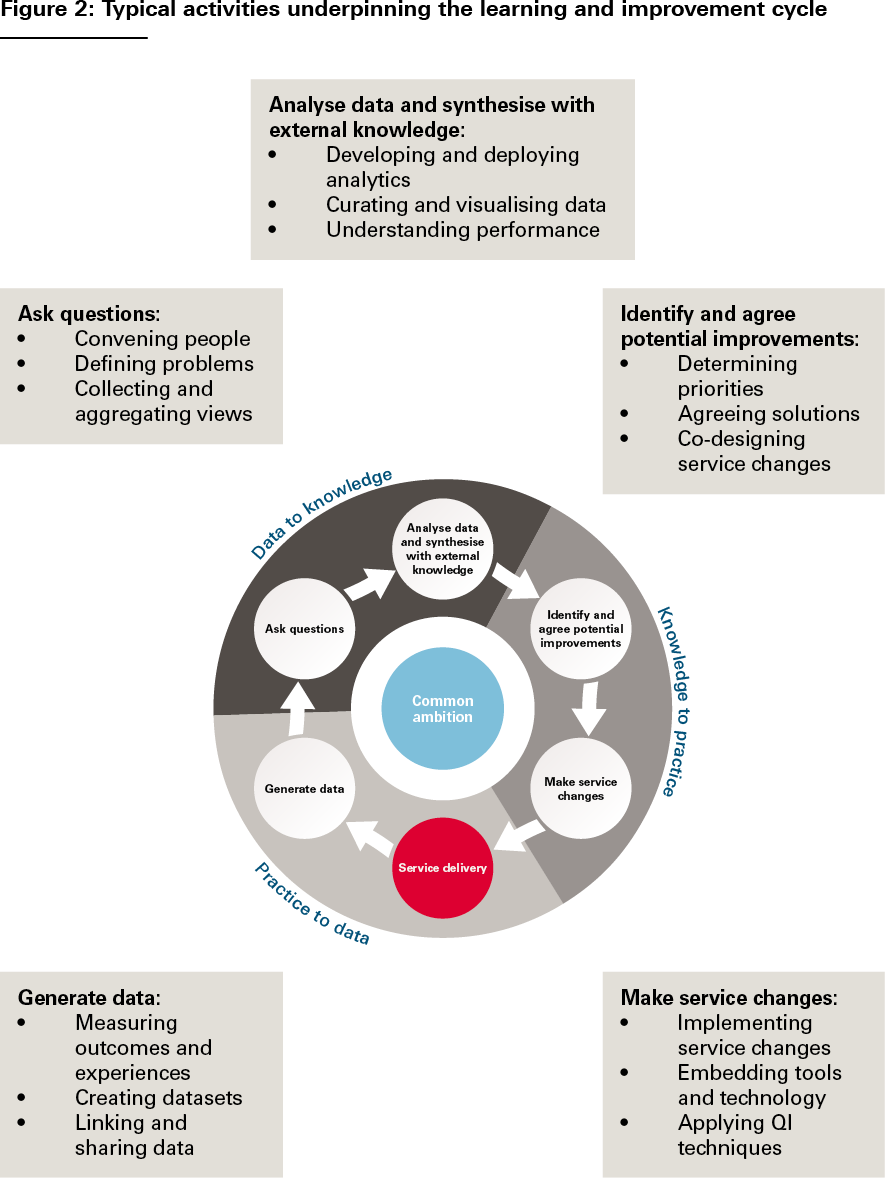
Source: The Health Foundation’s Insight & Analysis Unit
However, something the classic tripartite LHS schema does not always make explicit is how values and intentions get into the cycle – how questions get asked and priorities for improvement get determined. And this is a more fundamental issue than just the initial selection of an improvement ambition – the need to ask questions, gather and aggregate views, reconcile differences, and make judgements and course corrections is an intrinsic part of the learning process. For that reason, we think that it is important to consider problem definition and solution design as explicit parts of the learning and improvement cycle – signified in Figures 1 and 2 by the actions ‘ask questions’ and ‘identify and agree potential improvements’. Indeed, as we will discuss further later, it is these fundamentally human processes of convening, interacting, deliberating and making decisions that make the social infrastructure of LHSs just as important as their technical infrastructure.
Differences in the way each stage of the learning and improvement cycle happens can be another important source of variation between LHSs. Each stage can differ in scale and intensity (appropriate to the LHS’s goals) – that is, in the depth and granularity of the work going on, the number of people involved, the timescale, the cost and so on. For example, the data analysis involved in a learning and improvement cycle could range from reading a patient feedback form to a lengthy research study involving novel and complex analytics, while the service changes could range from putting up a sign in a waiting room to redesigning a whole health care pathway.
The scale and intensity of different stages of the learning and improvement cycle will therefore greatly affect what the LHS looks like in practice. In particular, the greater the scale or intensity required, the more likely it is that different individuals will lead different aspects of the cycle, or that these stages will happen in different environments. While at the smallest scale, the stages of an LHS could be executed by a single individual in a single environment (for example, a clinician using real-time feedback in a mobile app to optimise their practice), at the other end of the spectrum might be a learning and improvement cycle that involves lengthy research projects, specialist engagement exercises, the lab-based development of new technology or data tools, or the cross-organisational implementation of new clinical pathways.
In summary, variation in the three aspects of LHSs outlined here – the nature of services provided, the nature of the learning community and improvement ambition, and the scale and intensity of each stage of the learning and improvement cycle – makes many different types of LHS possible. Figure 3 illustrates some of this diversity, using a selection of the case studies presented in this report.
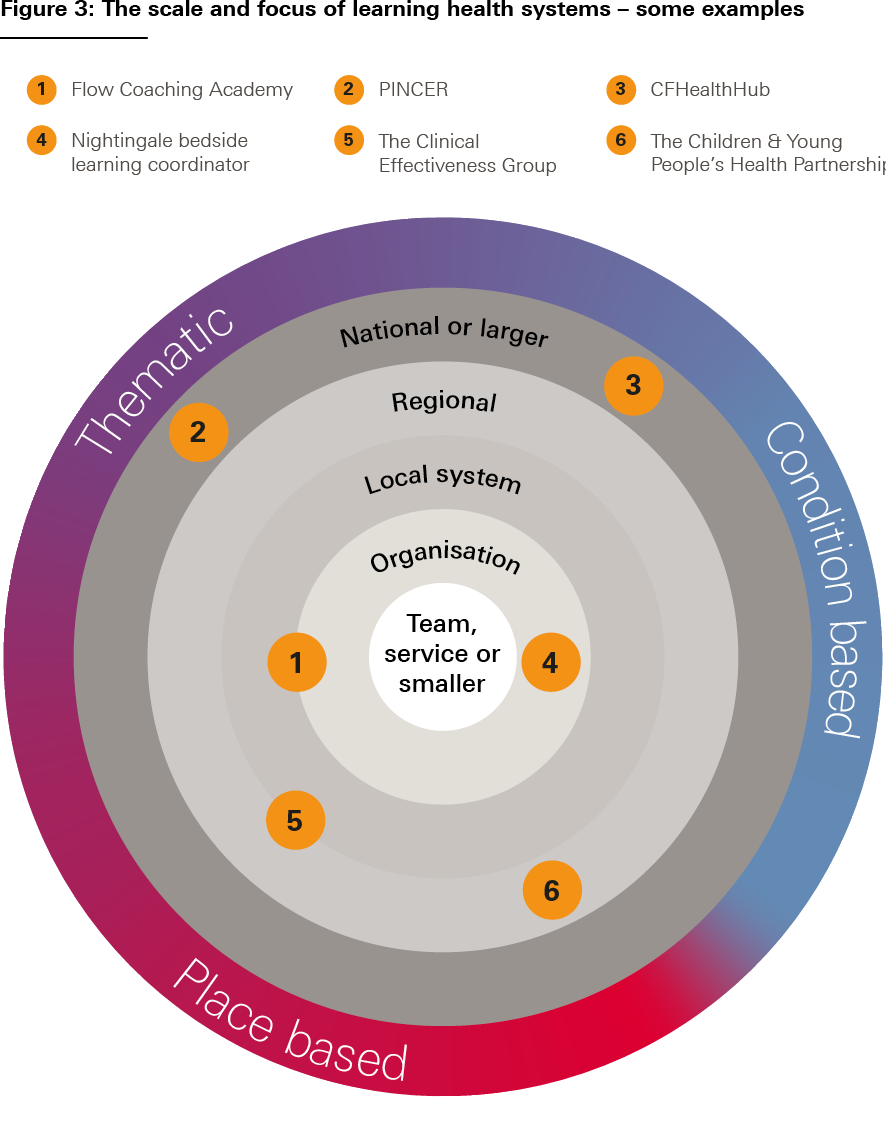
Source: The Health Foundation’s Insight & Analysis Unit
These six case studies are presented at the end of Chapter 1.
1.2. The assets underpinning learning health systems
Another approach to thinking about LHSs is to consider the capabilities and infrastructure that typically underpin them – in other words, going beyond thinking about the activities of the learning and improvement cycle to consider the assets on which these activities rely.
These assets, illustrated in Figure 4, include:
- data, data analytics capability and research capability, including skilled researchers and analysts
- technology, including data platforms, tools and systems, as well as an organisation’s wider digital maturity
- learning communities and networks, along with mechanisms, spaces and support for convening, deliberating and sharing knowledge
- improvement capability and culture, along with resources to enable the planning, design and implementation of improvements to care.
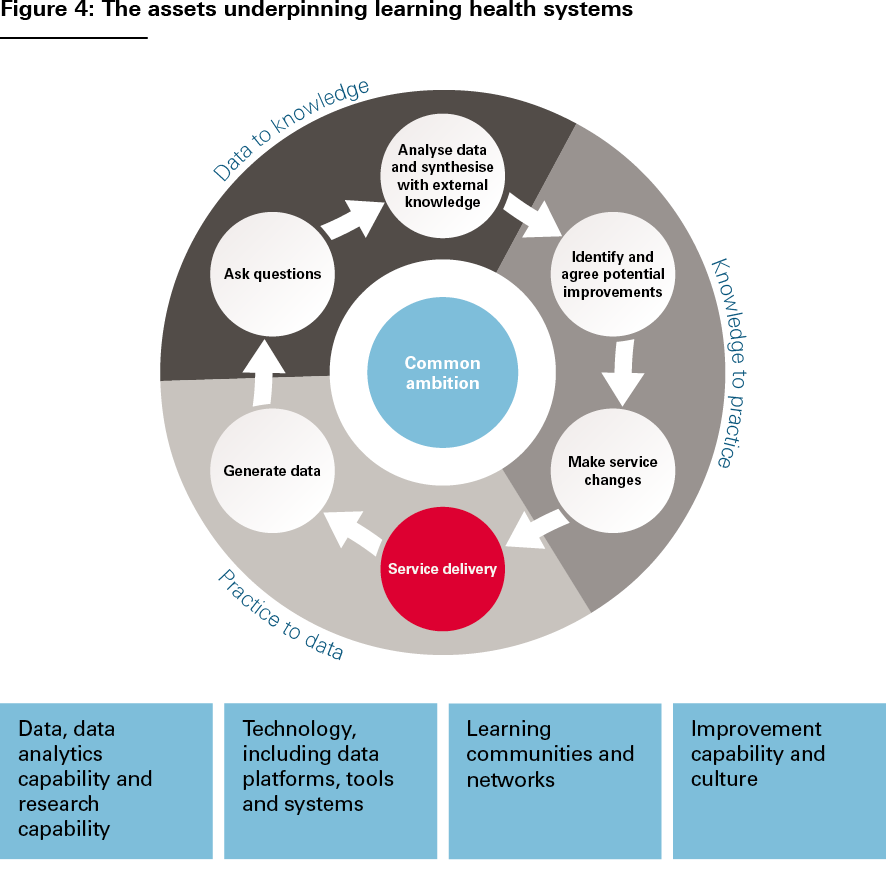
Source: The Health Foundation’s Insight & Analysis Unit
As the scale of an LHS increases, these assets will tend to become more visible and significant. And, of course, they are not static: they will develop and mature over time with successive iterations of the learning and improvement cycle and successive projects. And there is a need to continually nurture them.
Importantly, a provider or learning community might exhibit some of the components of an LHS but not all of them. And it is worth emphasising that the activities, capabilities and infrastructure required for successful LHSs are valuable in their own right, even when they are not being used as part of a full LHS.
Focusing on these kinds of assets and how they can be successfully developed should therefore be an important aim in itself. In practice, when thinking about how to support the development and evolution of LHSs, rather than trying to create entire LHSs in a single step, it can be more effective to focus on developing one or two components first – for example data analytics or improvement capability. In many cases, it will be about identifying what components are already in place and building on these. LHSs are not ‘all or nothing’ in this respect. Nevertheless, ultimately, it will be by bringing all these different components together and ensuring they are working in partnership that the LHS will become more than the sum of its parts.
Furthermore, there are other important elements of learning infrastructure in health and care that complement the concept of LHSs described here, and on which LHSs rely to connect and share learning (see Box 3 for further discussion).
Box 3: The relationship between learning health systems and other types of systematic learning in health and care
LHSs as described here are only one way in which systematic learning and improvement can happen in health and care. Other approaches exist that are not necessarily provider centred, nor involve learning and improvement cycles, nor even focus on a particular improvement ambition. Examples include the role of networks in spreading innovation – for example, Q, a community of thousands of people across the UK and Ireland collaborating to improve health and care – or peer learning through clinical communities. These kinds of wider approaches may use similar infrastructure and capabilities as LHSs (networks, data, improvement capability and so on), but they often go beyond the reach and focus of individual LHSs.
An exploration of these broader approaches to learning is beyond the scope of this report. But it is worth noting that they may play a very important role in helping to create an environment in which LHSs can flourish. While the endogenous aspect of LHSs (driving change from within) is one of their strengths, it does mean there is a need for linking mechanisms between them. Without this, there is a risk of siloed improvement efforts.
These broader approaches can be particularly important in bridging between different LHSs – helping them learn from each other and tackle unwarranted variation between different providers. They may also provide critical pieces of infrastructure on which LHSs rely, for example, platforms for research and consultation like Thiscovery (see case study 13 in the next chapter). So, LHSs as described here should not be considered in isolation from the health and care system’s wider learning infrastructure.
1.3. How learning health systems can help
Why should providers and learning communities be supported to adopt an LHS approach?
First, LHSs can support the delivery of externally led change through national programmes by providing the means to implement changes, test them and iteratively adapt and refine them, working with the very patients and staff the changes apply to. For example, in England, there are significant opportunities to develop LHS capabilities within integrated care systems as a way of supporting the successful adaptation and embedding of new pathways and models of care. In this guise, LHSs can be thought of as sophisticated ‘implementation mechanisms’ that can help deliver national priorities for service transformation.
But LHSs also matter because they are critical for enabling locally led service change. Many of the challenges in health and care cannot be solved by top-down change programmes alone. And while local systems face common problems and many solutions are generalisable, there are also problems and solutions that are specific to individual contexts, which those closest to them will need to diagnose and solve.
By creating the capability to learn and improve from within, LHSs can turn providers into ‘engines of innovation and improvement’, driving improvement in a way that is not reliant on national initiatives or investment. And over the long term, endogenous, continuous improvement has the potential to achieve more than a series of centrally led improvement initiatives and may in many cases be more effective in achieving sustainable quality and efficiency gains.,
Another reason for the growing currency of LHSs is that they are an important way to capitalise on the increasing availability of data and analytical tools. In short, our ability to learn has never been greater. Crucially, developments in data and data analytics are giving providers themselves the power to gain insights about pressing challenges and how to solve them, reducing the need for external analytic capability. The increasing sophistication of technologies such as artificial intelligence also presents further significant opportunities for data-driven service improvement.
National policy has been slower to focus on LHSs than other drivers of health care improvement (such as targets, incentives and competition). However, interest in LHS approaches has grown over the past decade as the limitations of these more traditional, top-down policy levers have become apparent. In England, for example, the 2013 Berwick review set out a vision for the NHS to become ‘a system devoted to continual learning and improvement of patient care’ and this led to the government proposing that the NHS should become ‘the world’s largest learning organisation’. More recently, the 2021 Integration and Innovation White Paper contained the ambition of ‘accelerating [the system’s] ability to learn, adapt and improve’, while NHS England have argued that integrated care systems should become ‘consciously learning systems’. Meanwhile, Healthcare Improvement Scotland sees the development of ‘human learning systems’ as a key part of its approach to quality management. So there now appears to be acceptance at the national level that building a culture of continuous learning and improvement is essential for improving quality, efficiency and effectiveness.,,
For all these reasons, LHSs are an idea whose time has come. Not only are there increasing opportunities to deploy them and increasing interest from national policymakers, but we will not be able to solve the huge challenges that services are facing adequately unless we fully exploit the potential of providers to learn and improve. Box 4 explores where the greatest potential for further development might lie.
The chapter concludes with six case studies from the UK of LHSs of varying scale and focus. And to gain insights from other countries where LHS approaches are used, Box 5 gives three international examples.
Box 4: Development opportunities for learning health systems – what our respondents said
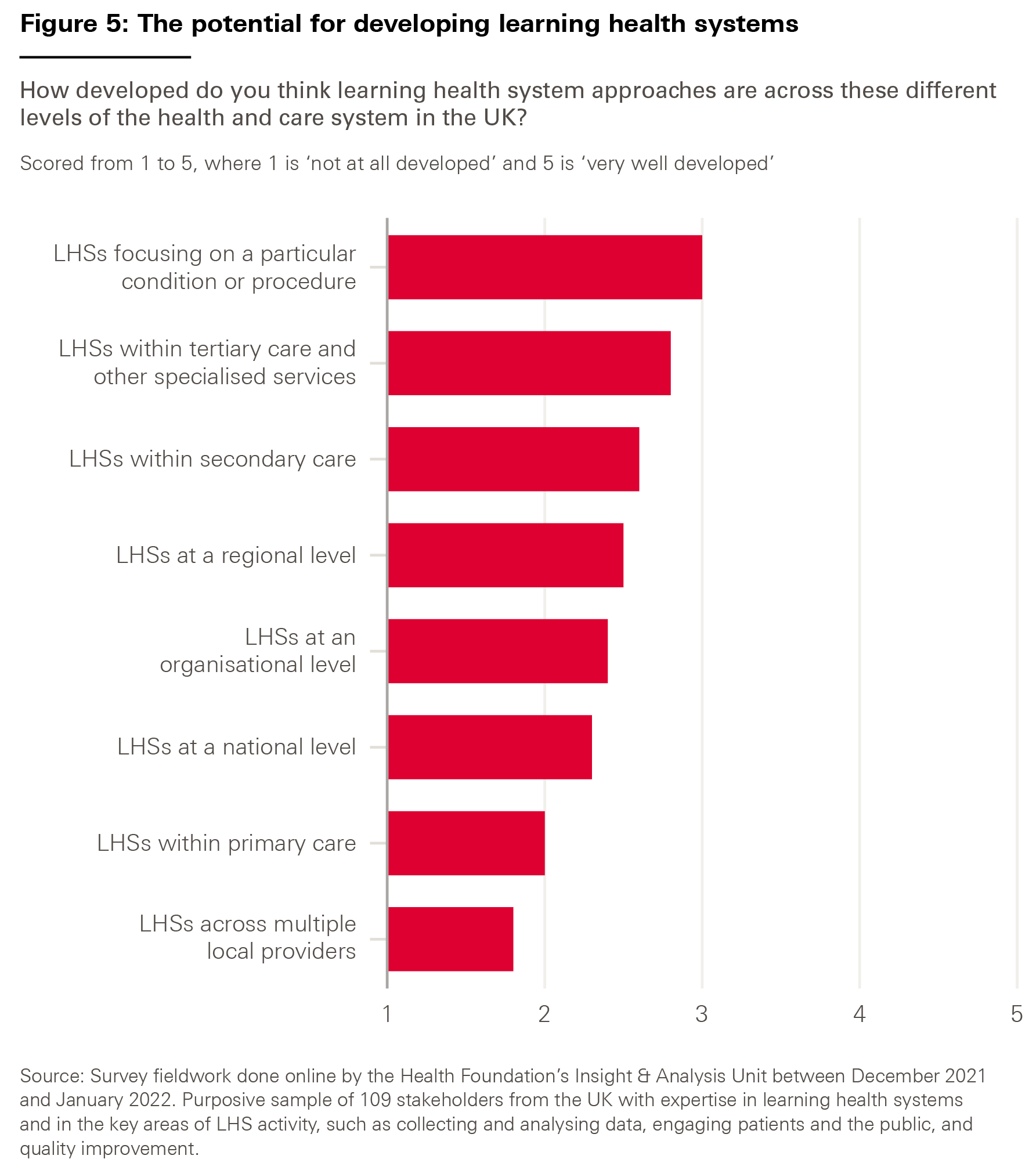
Given the diversity of possible types of LHS, we asked our survey respondents how developed they thought LHS approaches currently were across different levels of the health and care system – with an eye to understanding where the greatest potential for further development might lie.
The results, shown in Figure 5, suggest that LHSs have scope for development at all levels. But respondents felt that LHSs across multiple local providers, such as integrated care systems and provider collaboratives, were the least developed – perhaps unsurprisingly given the nascent state of these structures.

The effective movement of patients between departments and organisations, along pathways of care, and around the wider health and care system, is an essential part of delivering safe, timely and high quality care. Poor flow is a major contributing factor to adverse outcomes, readmissions and higher mortality rates, whereas good flow can improve outcomes and waiting times, reduce duplication and improve efficiency.
Set up by Sheffield Teaching Hospitals NHS Foundation Trust in 2016, the Flow Coaching Academy empowers teams to improve flow through a common purpose, language and quality improvement method. Through open, inclusive and non-hierarchical safe spaces called ‘Big Rooms’, teams collaboratively identify, develop and test local solutions informed by qualitative and quantitative data. Critically, each Big Room starts with a patient story to make sure their voice is a central part of the process – whether through a clinician telling a patient story or inviting patients to Big Room meetings.
The Flow Coaching Academy’s Roadmap for Improvement and ‘5Vs Framework’ underpin each Big Room, which provides a way for teams to assess a pathway and develop a shared understanding. Flow coaches, who have undertaken a one-year action-learning programme to develop relational and technical skills, including data analysis and coaching, work with teams to identify and achieve sustainable improvements to care within and across pathways.
The Flow Coaching Academy has delivered training to nearly 400 coaches from NHS trusts, clinical networks, charitable organisations and health boards across the UK. It has developed a network of local academies and training is currently taking place in Northumbria, Lancashire & South Cumbria and Sheffield.
The Big Room approach shows the importance of creating a learning culture where teams have the tools, opportunity and time to collectively define and implement improvements to service delivery. Part of the success of the Big Room, which emphasises that improvement is ‘20% technical and 80% relational’, is the focus on building multidisciplinary teams and a shared understanding, empowering all members to contribute.
Coaches encourage teams to take ownership of both the learning and improvement process and the data that inform it, which helps to develop better relationships across professional disciplines, including between clinical staff and data analysts. This is essential in building understanding of service performance.
Case study 2: PINCER – a pharmacist-led intervention to reduce medication errors
Medication errors, such as mistakes with prescriptions, preparation or dispensing, occur more than 237 million times a year in England. While most are minor, in an estimated 1.8 million cases these medication errors could lead to serious patient harm.
Researchers at the Universities of Nottingham, Manchester and Edinburgh developed PINCER, a pharmacist-led intervention that combines clinical audit tools with quality improvement methodology and educational outreach. Through the PINCER online resource centre, pharmacists can download searches to run on GP clinical systems that identify patients at risk of medication error. Pharmacists can compare their data to other practices across the country and then work with practice teams to improve prescribing processes and reduce potential harms.
PINCER goes beyond simple feedback tools by providing training through action learning sets that give participants the resources and skills needed to drive improvement and embed changes into everyday practice. Pharmacists develop skills in using quality improvement tools and strategies, root cause analysis, action plan development and delivering feedback. The action learning sets model has also provided participants with informal peer networks to support continuing development. More than 2,350 health care professionals have now been trained to deliver PINCER, including 1,785 pharmacists.
Supported by the Health Foundation and all 15 Academic Health Science Networks, the initiative, led by PRIMIS at the University of Nottingham, has now been adopted by more than 40% of GP practices in England through a social franchising model. This model has given individual localities the flexibility to tailor the intervention to their needs, which has been critical to its successful scaling. As a result, more than 220,000 at-risk patients have been identified, and analysis of follow-up data from 1,677 practices has shown a reduction of 32% in the number of patients at risk of hazardous prescribing associated with gastrointestinal bleeding – a common cause of medication-related hospital admissions.
Case study 3: CFHealthHub – a digital learning health system
Around 15 million people in England are living with at least one long-term health condition, accounting for 70% of health and care expenditure. However, it is estimated that up to half of all medicines prescribed in the UK for long-term conditions are not taken as recommended, with poor adherence to medical treatment having both a personal and an economic impact.
For the 10,600 people living with cystic fibrosis in the UK, daily inhaled medicines are vital for staying healthy, but only around 36% of people with cystic fibrosis are fully adherent to their complex treatment plans. To address this challenge, CFDigiCare, a collaboration of clinicians and people with cystic fibrosis, developed CFHealthHub – a digital LHS that seeks to optimise cystic fibrosis outcomes by creating a national community of practice that uses data to improve care.
Through a digital platform co-designed with users, people with cystic fibrosis can track their progress by accessing real-time medication data captured by their Bluetooth-enabled nebuliser. The CFHealthHub mobile app shows these data through accessible, colour-coded graphs that give feedback on treatment-taking.
Users can also choose to share the data with their clinicians, who then work with them to support behaviour change, identify barriers to effective treatment and talk through evidence-based strategies for overcoming them. A 19-centre randomised control trial showed that CFHealthHub increased adherence to treatment while reducing the burden and effort of self-care.
As of May 2022, CFHealthHub is used by 60% of adult cystic fibrosis units in England, creating a learning community of clinicians, managers, pharmacists and allied health professionals who are sharing their learning and best practice. Using the real-time automatic data capture of CFHealthHub, this community of practice is able to understand how well the system is supporting people with cystic fibrosis.
This has led to, and provided the infrastructure for, several linked, systems-optimisation workstreams. For example, the National Efficacy-Effectiveness Modulator Optimisation programme is carrying out a real-time health technology assessment of new medication which can significantly improve lung function, which is able to use data from 1,000 participants. The CFHealthHub has also shown how data gathered by technologies can be built into care, without burdening the patient or clinician, and how they can be used to both support system learning and improve personalised support for people with long-term health conditions.
Case study 4: Nightingale bedside learning coordinator
During the onset of the COVID-19 pandemic, NHS England set up NHS Nightingale Hospital London (the Nightingale) as a temporary facility in an east London convention centre to cope with the rising number of critical care patients in London. The novel setting, set up quickly with newly formed teams, meant the Nightingale had to manage significant risk and potential human error. In light of the knowledge gap surrounding COVID-19 and the need for rapid implementation of learning about the disease, the Nightingale was purposefully designed to be an LHS. The LHS approach enabled the Nightingale to rapidly make decisions backed by data and evidence to improve the delivery of care, quickly monitor the impact and make iterative adjustments where necessary.
A key component of the LHS involved gathering staff insights and ideas for improvement. The bedside learning coordinator role was developed as a mechanism to gather these insights rapidly and continuously without creating a burden for staff. The role involved:
- capturing staff insights into what was and was not working
- rapidly feeding these insights back to the leadership teams to review and agree how to respond
- implementing agreed changes as appropriate
- enabling robust feedback loops.
Staff from a diverse range of professional backgrounds (both clinical and non-clinical) undertook bedside learning coordinator shifts to give a broad set of perspectives and insights.
Insights captured were triaged into three areas: fix (requiring immediate action), improve (needing suggestions for better ways of doing things) and change (requiring substantial changes). Bedside learning coordinators worked with a central quality and learning team to triangulate insights from the bedside with other data sources, such as incident reports, team debriefs and performance dashboards as well as external evidence, to inform decision making and implement required actions as appropriate. In addition, as well as external evidence they carried out focused audits to confirm that implemented changes were successful, satisfactory to staff and sustainable. One example of this in action was the identification of mouth care as an area for improvement. Following concerns that staff had raised, a speech and language therapist completed a bedside learning coordinator shift to give specialist insight and recommendations. These were then adopted as standard operating procedure.
The Nightingale demonstrates that health care staff often have rich insights and ideas for improvement (including how to improve patient care, workplace efficiency and staff wellbeing), which, when analysed alongside other routine data sources, can support improvement work. The bedside learning coordinator role provides a mechanism to gather these insights, as well as giving staff a greater voice and empowering them to deliver tangible improvements as part of a wider LHS.
Since the initial pilot, several other large NHS organisations have adopted the bedside learning coordinator concept.
Case study 5: The Clinical Effectiveness Group
Data sharing between organisations within health and social care is often disjointed, leading to limited sharing of learning and the duplication of work between providers. As general practice moves to a model where bigger operational units – such as integrated care systems, primary care networks and GP federations – support service users with more integrated care, there is an opportunity to pool learning to support continuous improvement as part of an LHS.
The Clinical Effectiveness Group (CEG) at Queen Mary University of London is an academically supported unit that facilitates data-enabled improvement for 272 north-east London GP practices, serving 2.2 million patients. It brings together people from a range of disciplines, including clinicians, data analysts, informaticians, academic researchers and a team of facilitators who conduct around 300 GP practice visits a year.
The CEG builds standardised data entry templates that GP practices use to enter high quality data into their patient records at the point of care. Its software tools, searches and on-screen prompts then turn these data into actionable insights within the practice, for example to stratify patients by risk or to support self-reported measurements such as home blood pressure recording.
The CEG’s cardiovascular disease tools have contributed to improvements in blood pressure control, statin use and the management of other associated long-term conditions in the local population, with pre-pandemic performance among the highest in England. For example, pharmacists in the London Borough of Redbridge, in collaboration with St Bartholomew’s Hospital, are using one such tool – APL-CVD (Active Patient Link tool for Cardiovascular Disease) – to improve statin prescribing and identify suitable patients for a new drug that reduces cholesterol.
CEG analysts also create interactive dashboards showing performance across the region, allowing for the identification of areas requiring improvement. The CEG uses this evidence to design and deliver local guidelines and quality improvement programmes to reduce unwarranted variation in outcomes. The most recent is a programme to reduce inequalities in childhood immunisations. The CEG has championed GP recording of self-reported ethnicity to support the identification and reduction of health inequalities. The dashboards similarly reflect information on a range of equity indicators that local authority public health teams use to inform local initiatives.
Evaluation of the CEG identified key contributors to its success including:
- access to high quality coded GP data from across north-east London
- trust and credibility in its use of data
- engagement with local clinicians and health care providers
- the expertise of its clinical leads.
The CEG’s approach has put health data into practice to build an LHS in north-east London. The team is now working with other integrated care systems in London to support this approach in other areas as part of the London Health Data Strategy.
Case study 6: The Children & Young People’s Health Partnership
Research shows that some health systems are struggling to keep pace with the changing health needs of young people, and wide inequities in health remain among this group. With more than 180,000 children and young people living in the densely populated, diverse and fast-growing London boroughs of Lambeth and Southwark, an integrated approach to the delivery and coordination of care for this rapidly evolving population is essential.
The Children & Young People’s Health Partnership (CYPHP), hosted by Evelina London Children’s Hospital and part of King’s Health Partners, is a population-level LHS aiming to deliver better health for children and young people. Bringing together providers, commissioners, local authorities and universities, the CYPHP collaborates on taking care into the community, uncovering unmet need, and targeting care through technology and data-enabled early identification and intervention.
One of the CYPHP’s focuses is asthma. Data are gathered from several sources, including biopsychosocial data through a patient portal, routine clinical interaction data, data on wider determinants of health such as poverty and air quality, and data gathered through research that patients can opt into through the patient portal.
The team of clinicians, managers and researchers then translate these data into action by using them to make personalised decisions about patient care, support decisions on triage and inform what packages of care might be needed. The data are also used to inform population health management approaches by identifying which geographic areas have the greatest need, enabling earlier intervention.
The data are also being used for wider quality improvement and research activity. For example, through local test beds, the CYPHP is using a pragmatic but rigorous approach to evaluation by running randomised control trials alongside service evaluations that can quickly provide evidence to clinicians to support continuous improvement.
The CYPHP has demonstrated impact through a service evaluation, which showed improved health outcomes and quality of care as well as reductions in emergency department contacts and admissions. Our interviews with the team highlighted that by understanding population need through data, it is possible to deliver care that is proportionate to need and that can therefore help reduce inequalities in access to care among children, alongside reduced associated costs.
Box 5: International examples of learning health systems
While the case studies and examples featured in this report are from the UK, it is worth noting that there are many instances of LHS approaches being taken in other countries. Below we highlight three examples.
ImproveCareNow, United States
ImproveCareNow was set up to improve care for children and adolescents with inflammatory bowel disease in the US, which had seen significant variation in terms of both diagnostic testing and treatment. Through the setting up of a ‘collaborative learning network’, ImproveCareNow brought together a community of clinicians, researchers, patients and parents to use routine data for research and continuous improvement. All patients with inflammatory bowel disease within the network are now enrolled in a single patient registry, allowing ImproveCareNow to assess the impact of improvements on outcomes. Since its inception in 2007, ImproveCareNow has seen remission rates increase from 55% to 77%, and the network has grown to provide care for more than 17,000 patients across 30 states of the US.
Swedish Rheumatology Quality Registry, Sweden
By building on routine care data and their existing ‘outcomes dashboard’, clinicians in Sweden’s Gävle County were able to improve outcomes for patients with rheumatic diseases, going from having the worst outcomes in the country to the best. Patients were supported to use their data at home to understand when they might be out of remission. They were also able to use the information to control their care through an ‘open–tight’ model: when patients were doing well, they were ‘open’ to visiting a clinician if they felt they needed to and were supported to self-care, but if they were not doing as well, they would be ‘tightly’ cared for until that care was no longer needed. This approach both decreased unnecessary attendances and encouraged self-management approaches that saw outcomes improve substantially.
Johns Hopkins Medicine’s ‘learning and improving system’, United States
In recent years, Johns Hopkins Medicine has introduced an organisation-wide LHS approach that seeks to break down traditional silos between research and practice in order to improve patient outcomes and reduce waste. Bringing key leaders together around a clear and compelling, patient-centred purpose, its ‘learning and improving system for quality and safety’ is underpinned by a wide-ranging learning community, but with clear links to management for accountability. By aligning its goals and strengths across a broad range of stakeholders, the approach has seen significant improvements in a range of areas, including reductions in surgical-site infections of more than 50% and significant improvements in patient feedback.
Four key areas that can support progress on learning health systems
2.1. Introduction
As highlighted in Chapter 1, there is growing interest in learning health systems (LHSs) as a vehicle for improving care quality and service delivery. But given that LHSs are multifaceted and can be highly complex, it can be difficult to know where to begin. So, what should policymakers, and organisational and system leaders, be focusing on to support the development and spread of LHS approaches?
Our research and engagement with expert stakeholders highlighted four key areas where targeted action could help to advance the use of LHS approaches:
- learning from data
- harnessing technology
- nurturing learning communities
- implementing improvements to services.
These four areas, which link to the assets and capabilities underpinning LHSs as described in Chapter 1, form key lines of enquiry in this chapter. Specifically, we discuss each of the four areas in turn, setting out the current context, opportunities and challenges. In each case, we also report the results of our stakeholder survey to identify which of the challenges we describe currently require the most attention.
2.2. Learning from data
2.2.1. Key issues and opportunities
The potential of data for learning health systems
Better use of data has huge potential to improve the quality, safety and cost-effectiveness of care and address unwarranted variation, across the whole health service. The data can be quantitative or qualitative and be drawn from many sources. As discussed in Chapter 1, one of the characteristic features of an LHS is that it uses data generated from routine care delivery – whether clinical, operational or patient-reported. However, LHSs are of course not restricted to using these data and can draw on data from a wide range of sources, such as patient surveys, research and clinical trials, and increasingly data from technology such as smartphones and wearable devices (wearables), like smart watches and medical technologies, that are worn by individuals to track, analyse and transmit data.
Within quality improvement work, it is not uncommon to hear people distinguish between ‘data for care’ and ‘data for improvement’. But a more useful approach is to distinguish between the primary purposes of health care data (delivering direct clinical care) and the secondary purposes of such data (such as research, population health management and improvement). Both uses of data support the work of LHSs, in particular in the design of services and helping to build understanding about how they are performing and how they can improve.
As the volume, breadth and quality of data increase, so too do the opportunities for learning and improvement. Wider sources of data, such as data generated by smartphones and wearables, and data on the social determinants of health, are increasingly playing a valuable role by allowing us to build a more holistic picture of our populations. SAIL Databank (case study 7), a Trusted Research Environment that contains data about the whole population of Wales, shows how this wide range of data can be brought together.
Combining and analysing data from different sources can generate new clinical evidence. The Informatics Consult project (case study 8) shows how this can be done. It is particularly important for supporting treatment decisions for patients with complex health needs, where traditional forms of evidence are lacking.
Understanding the health and needs of people and their communities
The move towards better collaboration between health and care providers and commissioners – through, for example, health boards in Scotland and integrated care systems in England – provides an opportunity to improve the health of their populations. By integrating infrastructure, developing standards for interoperability (the ability of two or more systems to exchange information and use that information) and working collaboratively, these organisations can identify where within their populations the greatest needs lie, and work together with stakeholders, including members of the community, to design data-driven interventions to address those needs.
There are particular opportunities for better use of patient-reported data. But while the NHS collects a large amount of patient-reported data, they could be used much more effectively. This is where an LHS approach can help because it centres on the ambition of putting knowledge into practice.
Data collected from communities offer the opportunity to help health care services better understand what matters most to them. This includes national collections such as patient and service user experience surveys and patient-reported outcome measures, as well as local routes, from focus groups to service user surveys. Initiatives such as the Networked Data Lab, which the Health Foundation leads, are linking these datasets together to build a more complete picture of the relationships between the wider determinants of health, health needs, service use, patient pathways and health outcomes.
Data about particular health conditions, including on diagnosis, treatment and outcomes, also offer potential for LHSs – including the possibility of creating LHSs around particular conditions. Clinical registries and clinical audits, for example, allow comparisons between multiple sites, reveal variation in processes and outcomes, and identify where improvements can be made.
Making data easy to understand and actionable
While the growth in the volume of data means there is ever more knowledge that can be generated, the amount of data already exceeds what people working in health care can assimilate. There are other challenges too. Often data are not presented in a useful way, and long time lags (between data collection and use) mean that they can be too old to have relevance to health care decisions today. For example, as patient registries become able to collect data on patients’ priorities in real time, incorporating patient-reported data with clinical data, they could become a critical part of the infrastructure of LHSs where patients, clinicians and scientists work on service improvements together.
So, there is considerable scope to improve both the curation of data (the organisation and management of data from various sources) and the visualisation of the data – along with how and when the data can be accessed, so that health care professionals can see relevant insights in a timely and digestible way to support their decision making. For example, the Clinical Effectiveness Group at Queen Mary University of London (case study 5) has created interactive dashboards showing performance across GP practices in north-east London, allowing for the identification of areas requiring improvement.
Elsewhere, academics at the University of Edinburgh have developed a dashboard to support GPs to improve care for people with asthma across the UK (case study 9). The dashboard gives GPs weekly data on asthma indicators at their practice and shows how the data compare with those for other practices.
Building data analytics capability
Data analytics methods, tools and skills – including novel statistical models and data linkage – are a critical part of LHSs because they generate new knowledge and insights to support the learning and improvement process.
Our research and funding programmes at the Health Foundation show that the data analytics capability that exists in the health and care system is both underdeveloped and underused.
The impact of data analysts could be increased considerably through training and professional development, and through better access to the software tools required to generate insights from data. Specialist analytical expertise can also help to develop data literacy in the wider health care workforce. For example, the Flow Coaching Academy (case study 1), a model centred on a multidisciplinary micro-team approach to improving service delivery, shows how this potential can be exploited. By bringing data analysts into multidisciplinary team meetings known as ‘Big Rooms’, and coaching clinical staff in how to interpret data, the model enables teams to gain a better understanding of how services are performing and, critically, where improvements could be made.
Evaluating improvements
Testing and evaluation – which rely on collecting the right data – are a critical part of an LHS to show whether changes made have led to improvements. This mostly happens as an integral part of the iterative learning process, with the data collected in subsequent learning cycles being used to assess the service improvements implemented in previous cycles.
There are promising ways in which testing and evaluation can be done that marry robustness with timeliness. For example, the Children & Young People’s Health Partnership (case study 6) shows how running different evaluation models simultaneously can effectively support the learning and improvement process. By running randomised control trials at the same time as service evaluations, the partnership can give evidence quickly to clinicians to inform care improvements, and to commissioners and managers who can make rapid decisions about embedding new services into ‘business as usual’, while building a robust evidence base for children’s health care more widely.
2.2.2. Challenges
While some health care services and systems have made great progress in collecting and using data, our research suggests there are a range of challenges that stand in the way of using data to drive continuous learning and improvement in the manner that is required for LHSs. Several of these challenges have been highlighted in the government’s recent strategy Data Saves Lives, which also sets out steps to make progress on many of them.
LHSs require the availability of high quality, actionable data on a range of issues, including performance, outcomes, experiences and processes. This has been a particular challenge for patient data as progress towards the digitisation of patient records has been slow. Where data are recorded electronically, they do not always meet quality standards, nor are they always useful. Data can be incomplete or captured incorrectly.
Stakeholders we spoke to during our research told us that there are significant issues surrounding data access and sharing, including information governance and data security, which can limit the work of an LHS. In order for a team to be able to access data, a range of approvals are required, which vary in number and complexity due to a lack of standardisation. In addition, the regulation of data often lags the rate of innovation and the data that it produces. Financial costs associated with data access can also hamper the ability to use data to learn and improve. Health and care data must be in a readable, actionable format to have value, which often relies on data system vendors curating and cleaning the information they hold, and they can then charge sizeable fees.
It can be difficult to know what approvals are required to access data and from whom. For example, innovators involved in the RADAR project (case study 10) told us how introducing the MyWay digital health app into some parts of England required data-sharing agreements with each data controller. This included individual GP practices, clinical commissioning groups and Caldicott guardians (who are responsible for protecting the confidentiality of people’s health and care information and making sure it is used properly) compiling population-level data for the area, which took an enormous amount of time and effort to achieve. Conversely, use of the platform in north-west London was far simpler as data access is dealt with at the integrated care system level, where there is a structure for data controllers signing off electronically, and a population-level dataset already exists.
Our experience of funding data analytics projects at the Health Foundation suggests that linking and integrating datasets can also be particularly difficult, not only because of technical challenges, but also because of the way in which increased data linkages can challenge processes of anonymisation. The number and complexity of processes required to link data can be particularly challenging. For example, in the LAUNCHES QI study (linking audit and national datasets in congenital heart services for quality improvement), run by University College London, which sought to improve services for people with congenital heart disease by linking five national datasets, the process took 2.5 years to achieve, requiring nearly 50 documents for the data application processes, which needed to be submitted 162 times in total. Stakeholders told us that there are also challenges with integrating datasets, and insights derived from them, within electronic health records and that integrating analytical tools into electronic health records can be both difficult and very expensive.
System interoperability – the ability of two or more systems to exchange information and use that information – continues to be a challenge in health and social care. With an assortment of different data systems, and a lack of common standards, it can be difficult to gather and aggregate the data needed to drive an LHS. A 2020 National Audit Office report on digital transformation in the NHS in England highlights that while interoperability has been a priority for policymakers since 1998, progress has been slow, and a lack of clarity on standards to encourage new suppliers to enter the NHS could make interoperability harder to achieve.
For health care services to learn about and design the most effective interventions, they require data that go beyond routine clinical data – for example, data on the social determinants of health. Yet access to a wide-enough range of data can be challenging and many types of information are not captured in datasets. For example, routine NHS data do not include data on those who do not access health services, nor do they include information about a patient’s health in between interactions with the health service – both of which could improve understanding of the drivers of ill health.
The disproportionate impact of the COVID-19 pandemic on some groups has rightly highlighted the importance of tackling health inequalities, but to do this requires data that are representative of all patient populations and free from bias, which is often not the case. This can be due, for example, to a failure to capture data on characteristics (such as ethnicity) accurately, a lack of representation of different demographic groups in research or a lack of access to technologies that capture data., Such factors can render datasets biased, which can lead to decisions that either do not bring about improvements to service delivery or – worse – risk adverse outcomes and experiences for some service users. This is a particular concern for data-driven health technologies such as those using artificial intelligence and machine learning.
Understanding Patient Data research shows that most people support sharing patient data for individual care and a high proportion of people support sharing patient data for research where there is public benefit. But making sure that patients and the public, and also the health care workforce, perceive the collection, sharing and use of data to be trustworthy can be a challenge. Transparency and open dialogue with the public are important ways of achieving this but are not always addressed sufficiently.
Data need to be formatted and ‘cleaned’ to allow them to be analysed. This can be laborious and time-consuming work, which often fails to get the attention and resources it requires and is often done on an ad-hoc basis, leading to duplication of data. Both this data curation work and the subsequent data analysis require specialist skills and knowledge, and while there have been moves to build this capability across the health and care system – including through NHS England’s work to develop a National Competency Framework for Data Professionals in Health and Care – more is required. Another related issue is more general health data literacy across the workforce, so that staff, including those in management and leadership positions, can interpret data appropriately.
We asked our survey respondents which of the data challenges highlighted in bold above they considered to be the most significant for adopting LHS approaches; the results are shown in Box 6. (For each part of our survey, we also asked our respondents to tell us whether there were any challenges we might have missed, but the responses suggested that there were no significant omissions.)
Box 6: Data challenges – what our respondents said
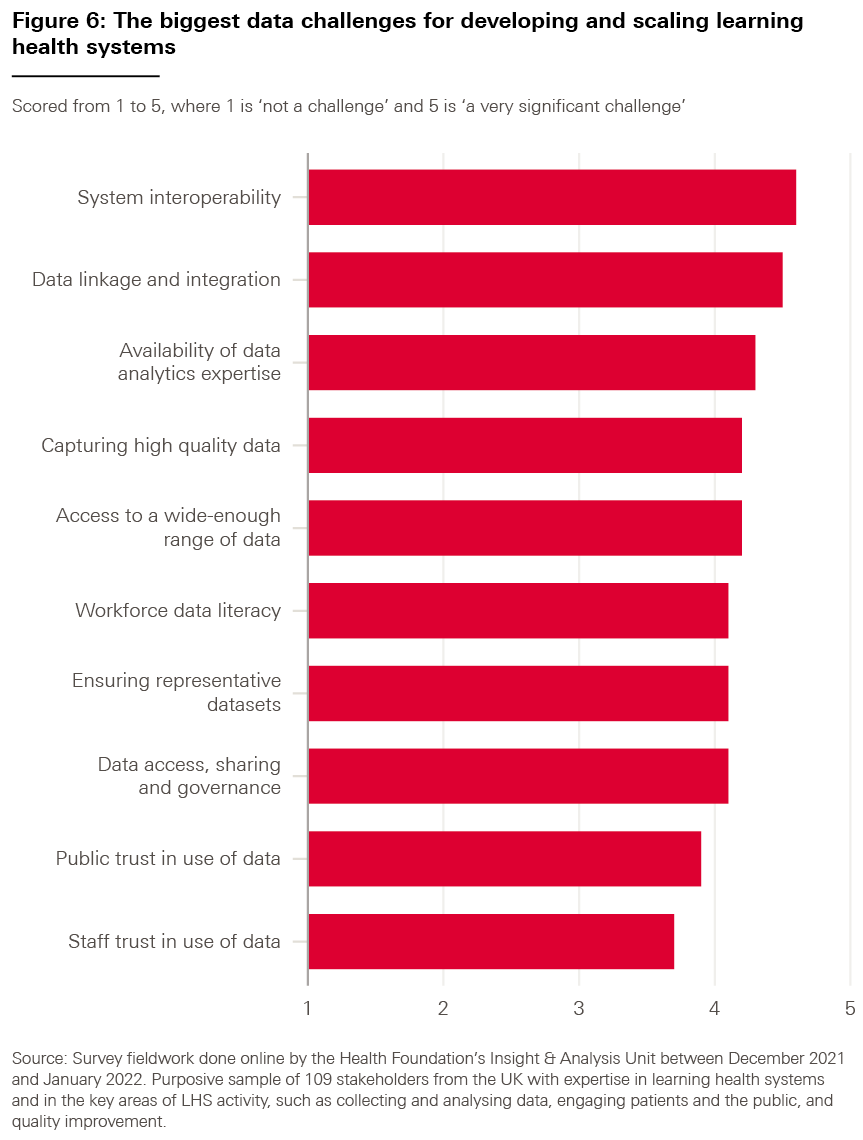
As shown in Figure 6, the results indicate that all proposed factors were thought to be a challenge to some degree, averaging a score of 4.15 out of 5 (with 5 representing ‘a very significant challenge).
However, the highest-ranked challenge was ‘interoperability between data systems, both within and across organisations’ (4.6), followed by ‘linkage between datasets and integration of these datasets with electronic health records’ (4.5).
Case study 7: The Secure Anonymised Information Linkage (SAIL) Databank
Established in 2007, the Secure Anonymised Information Linkage (SAIL) Databank is a Trusted Research Environment holding anonymised individual-level data for the whole population of Wales. One of the world’s first Trusted Research Environments and hosted within Population Data Science at Swansea University, SAIL was set up to use data gathered in health and social care delivery to better inform research, improve services and inform population health strategy. It includes data that clinical interactions and interactions with social and community services generate, allowing for analysis of links between health outcomes and social factors.
SAIL operates according to a ‘privacy-by-design’ model, which uses physical, technical and procedural measures to safeguard the data it contains, prohibiting the sharing of data outside the databank without special dispensation. Also, to gain access to data held in the databank, prospective researchers must undertake a two-stage application process, which an independent Information Governance Review Panel assesses.
Population Data Science created the Secure eResearch Platform (SeRP), which powers the SAIL Databank, and allows researchers from across the world to access data linkage services and a wide range of data to answer important questions with the use of analytical tools. By 2020, SAIL had more than 1,200 registered users and has been used to deliver more than 300 research projects, including the development of National Institute for Health and Care Excellence (NICE) clinical guidelines. Strong relationships with many partners – such as Digital Health and Care Wales, the Welsh government and Public Health Wales – underpin the SAIL approach, which has enabled the use of data to inform decisions in policy and practice, notably those made in response to the COVID-19 pandemic through the One Wales collaboration.
From the beginning, the SAIL team recognised that public trust in the handling and sharing of personal data would be critical to the databank’s success. This trust relies on several interacting factors such as cultural values, personal preferences and mass media influences. To gain public trust, the SAIL team developed a programme of public involvement and engagement to assess public opinion and gain input into policies and practices, which a consumer panel – including members of the public – oversaw. The consumer panel also advises on routes and methods to engage with the population, recommends how information can be shared with the general public and assists in reviewing proposals from researchers applying to access the databank. Projects that have used SAIL are shared on its website, including a description and a list of outputs, to make sure there is communication and transparency with members of the public and stakeholders.
Case study 8: Informatics Consult
A Health Data Research UK (HDR UK) and Health Foundation Catalyst project
While clinical guidelines play an important role in health care delivery, they are not always backed by a robust evidence base in the form of clinical trials and research. This is made more challenging as the number of people with comorbidities increases and the health needs of the population become more complex, with there being many situations where a treatment indication and contraindication coexist for one patient, for example patients with both heart failure and kidney failure. Given that treatment for one condition can have an adverse impact on another, and there is limited evidence for some treatment options partly due to randomised control trials frequently excluding patients with several comorbidities, it can be difficult for clinicians to determine the best treatment for their patients.
The growing availability of large datasets and the tools to analyse them provides an opportunity for improving decision making, particularly for patient groups for whom robust evidence does not yet exist. The Informatics Consult platform allows clinicians to select a health condition and order an analysis of large datasets to aid decision making for the specific patient in front of them. The platform employs automated approaches for creating analysis-ready cohorts using the ‘DExtER’ tool. Within hours, the platform returns easily interpretable clinical information – including on the potential benefits of treatment, prognosis and mortality risk – which can support more personalised treatment plans.
Drawing on population data contained within electronic health records, the platform presents analysis in a way that is understandable for clinicians and can be discussed with their patients. This is not always straightforward, though. For example, many rare conditions do not have specific clinical codes, which leads to challenges incorporating them into the platform.
The project team plans to test the Informatics Consult platform in four NHS trusts to support decision making with patients who have both liver cirrhosis and atrial fibrillation, where the use of anticoagulants can treat the latter while making the former worse. A pilot conducted using Informatics Consult generated new clinical evidence for patients in this group, showing that the initiation of warfarin was common, and may be associated with lower all-cause mortality and may be effective in lowering stroke risk. Surveys of clinicians using the platform showed that 85% found information on prognosis useful and 79% thought they should have access to the platform as a service.
By providing information on the prevalence of conditions as well as information on the safety and efficacy of a particular medication, it is hoped that this will stimulate further initiatives to generate new analyses for a wider range of prognostic outcomes. Given the rising trends of multimorbidity, especially in younger people, the Informatics Consult may contribute to the creation of a knowledge base generated from real-world datasets to address the current gaps in randomised control trials (arising from the exclusion of patients with comorbid conditions).
Case study 9: Towards a national learning health system for asthma in Scotland
Asthma is a significant cause of ill health and hospitalisation in the UK, costing an estimated £1.1bn and leading to 1,400 deaths every year. Around one in every 14 people in Scotland are currently receiving treatment for it and 89 in every 100,000 people are hospitalised each year due to exacerbations (increases in severity). Timely patient data is key to understanding and preventing exacerbations.
To address this challenge, researchers at the University of Edinburgh are working towards developing a national LHS for asthma that will support clinicians to identify and address modifiable factors that can contribute to exacerbations.
By harnessing routinely collected, anonymised data from the Oxford Royal College of General Practitioners Clinical Informatics Digital Hub (ORCHID), the team created an online dashboard for asthma that gives GPs weekly updates on how their practice compares with their network across several indicators.
Information provided includes comparative data on asthma prevalence, vaccination uptake, smoking rates, hospitalisations and body mass index measurements. This enables GPs to see changes in their practice, compare themselves to other practices and rapidly respond to better support people with asthma, prevent exacerbations and potentially prevent avoidable deaths.
In repurposing routine data to generate knowledge that can then be incorporated into clinical practice in real time, the LHS for asthma is one of the first applications of LHS approaches at a national level outside the US. The project champions innovative approaches to near real-time data visualisation, allowing health care providers to compare care and service quality to evidence-based standards and drive improvement.
While the impact has so far been limited due to the effects of the COVID-19 pandemic and workforce pressures, the researchers are now seeking further funding to develop complementary behavioural, motivational and organisational interventions that can tackle the barriers to using the data to make improvements. They are also going to develop a learning-based prediction model in order to create a personalised risk assessment tool to further support clinicians to predict asthma attacks and reduce asthma morbidity and mortality.
2.3. Harnessing technology
2.3.1. Key issues and opportunities
While not a necessary part of an LHS, technology can clearly play a very important enabling role, for example by:
- enhancing data collection and analysis
- accessing high quality information through electronic health records
- helping with clinical decision making
- supporting the design and implementation of improvements.
Increasing the use of digital health technologies is a priority for the UK’s health and care systems to support both service delivery and improvement. For example, NHS England’s service transformation plans are currently seeking to capitalise on the potential of LHSs to generate and embed knowledge and drive improvements in health and care.
Enhancing data collection and analysis
Digital technologies can support LHSs by enhancing the way health data are collected, for example through devices such as mobile phones, wearables and sensors. These technologies can enable the real-time collection and analysis of health data that may have previously been prohibitively expensive, intrusive or time consuming to collect or understand. For example, the CFHealthHub (case study 3) shows how data gathered through the routine use of Bluetooth-enabled nebulisers helps clinicians to understand how well existing care pathways are supporting people with cystic fibrosis. By integrating technologies into the existing care pathway, this example shows how data can be used to support system learning and improve personalised support for people with long-term health conditions. The use of digital health technologies such as these may also mean that the data collected is of a higher quality when compared with data inputted into a system manually. But it is important to make sure that those using these technologies are comfortable with using them, capable of using them and motivated to do so.,
In addition to collecting data, digital health technologies also often incorporate additional functionality such as analytics and artificial intelligence (statistical tools and algorithms that enable computers to simulate elements of human behaviour such as learning, reasoning and classification), which seek to maximise the value of the data. Technologies like natural language processing (a branch of artificial intelligence) can make complex data available to LHSs in a timely way by transforming large amounts of qualitative data into an analysable format. For example, it can analyse free text patient feedback, which can then enable a better understanding of what matters most to service users.
Accessing high quality information through electronic health records
Electronic health record systems have the potential to make sure that the right information is available at the right time. As longitudinal records of people’s health information, they are an invaluable resource for front-line teams needing to access an individual’s up-to-date health history and make decisions about their care and treatment. Collectively, they also contain information about the health of populations, which can inform decisions about what services and support are needed, both now and in the future, as well as show whether existing models of care are delivering the desired outcomes. For example, Northern Ireland’s Encompass programme is showing how health and social care records can be integrated and made accessible through a single system so that patients, service users and health and care staff can access timely information.
The information in electronic health records can also be used to help design evidence-based improvements to care. For example, Cambridge University Hospitals’ eHospital programme (case study 11) used insights from its electronic patient records to develop systems and alerts for the early identification of sepsis, which the team estimate led to a 70% increase in patients receiving timely treatment.
Helping with clinical decision making
Electronic health records can be enhanced through the integration of digital tools like clinical decision support systems. These systems can strengthen medical decision making by matching an individual’s electronic health data to a clinical knowledge database to make personalised care recommendations for the clinician’s consideration. This allows clinicians to access and apply continually evolving clinical guidance when it is needed. Clinical decision support systems are an example of how a technology can be used for both delivering care and enabling improvement – for example by incorporating machine-readable guidance that can be automatically updated and improved over time.
Several hospital trusts – such as Great Ormond Street Hospital for Children NHS Foundation Trust, Nottingham University Hospitals NHS Trust, University Hospitals Birmingham NHS Foundation Trust and Liverpool University Hospitals NHS Foundation Trust – have integrated clinical decision support systems into their electronic patient record systems. For example, University Hospitals Birmingham NHS Foundation Trust has used its electronic health record system to improve decision making in prescribing by:
- supporting clinician behaviour change
- minimising unintended drug omissions
- supporting decisions on dosing
- reducing the unnecessary use of blood products.
Clinical decision support systems have major potential for improving services and have recently been identified as a priority in NHS England’s transformation plans. They have been shown to reduce mortality, harm and life-threatening events. But they depend largely on the availability of ‘computer-executable knowledge’, that is, having clinical knowledge available in a format that a computer can apply, which can present a number of professional and technical challenges to implement.
These data tools and clinical decision support systems can also be enhanced through machine learning to support decision making. One example is risk prediction tools, which can support both individual-level care decisions and population-level strategies. For example, Project Breathe (case study 12) applied artificial intelligence to data from self-monitoring devices to predict cystic fibrosis flare-ups 10 days earlier than would have previously been detected.
Artificial intelligence technologies are also being built into clinical decision support systems so that their outputs are based on more complex data analysis, to improve the evidence base for clinical decision making. Artificial intelligence is also being used in platforms such as mobile health apps and risk assessment tools, which can be a way for people to quickly access, view and understand complex information in order to make decisions about their health care. For example, the RADAR project (case study 10) explored whether a digital platform could support people with type 2 diabetes to make decisions about their health by displaying their risk of complications based on their health data. It also allowed clinicians to understand whether and when to intervene earlier to prevent complications and avoid hospitalisation.
Supporting the design and implementation of improvements
Technology can play a role in explicitly supporting the process of designing and implementing improvements. For example, digital communication technologies such as research platforms like Thiscovery (case study 13), collaboration spaces like the FutureNHS collaboration platform and software repositories like GitHub can accelerate the pace at which ideas, evidence, tools and solutions can be shared. And they can bring together stakeholders from across diverse backgrounds and geographies to collaborate, discuss ideas and design improvements.
Technologies can also support implementation by replicating everyday situations and allowing improvements to be tested in safe, while realistic and immersive, environments through simulation technologies such as virtual and augmented reality.
2.3.2. Challenges
Developing, integrating and scaling up technologies within health care is a complex process, and our research has identified several challenges for using technologies to support LHS approaches. These challenges encompass many elements of the health care system, from infrastructure, workforce and skills, to funding, evaluation and trust.
Having adequate, underlying IT infrastructure is essential, in terms of hardware, software and internet access. A recent House of Commons Health and Social Care Committee report stated that providers will be unable to capitalise on the potential of technology while continuing to ‘struggle with basic IT infrastructure’. But despite a focus from national policymakers, digital maturity remains a significant issue, with one in five NHS trusts still relying on paper records.
The most recent data from NHS England’s Digital Maturity Index also show that there remains a significant disparity between organisations, meaning that some will require greater levels of support than others before they can maximise the potential of existing technologies and effectively adopt new ones. And with integration being a vital element of current reforms across the UK, efforts to improve digital systems will need to go beyond acute care to incorporate primary care, community care and social care providers too. Primary care providers have made significant progress on the digitisation of patient records, but wider challenges remain. Community and social care providers continue to struggle with system fragmentation, legacy IT systems and insufficient access to national funding. While the COVID-19 pandemic has accelerated the uptake of technology in out-of-hospital settings, in particular video conferencing, and reduced the number of ‘digitally novice’ organisations, many community and social care providers still require support with fundamental aspects of IT, including cybersecurity and data protection.
Securing funding for the development and deployment of digital health technologies also remains a challenge for NHS organisations looking to innovate, adopt and spread technology. Recent Health Foundation analysis suggested that the funding landscape is complex and skewed towards early-stage innovation – implying there would be benefits to more support for testing, adoption and spread. Challenges also exist for companies looking to finance innovative ideas. The high-risk nature of innovation, coupled with the long timeframes required for development and the need to deliver a return on investment, mean that many innovations succumb to the ‘valley of death’ between research and commercialisation, with half of digital health start-ups failing within their first 2 years.
Critical to financing innovations, as well as for their wider adoption and scaling, is generating sufficient evidence to show their safety and efficacy in real-world settings. But as discussed earlier in this report, generating this evidence is costly and complex, particularly for digital health technologies that develop at speed, and can be high-risk. Conventional methodologies, such as ‘gold standard’ randomised control trials, are rarely affordable for the health care technology market, which is dominated by small and medium-sized enterprises. They can also be limited in their ability to account for the complexity of many digital health interventions, whose contexts can vary considerably and continually evolve. Lengthy trials may also be unsuited to the rapid pace of digital innovation, with technologies likely to have been updated or even superseded by the time the trial is concluded. Prolonged, single evaluation approaches rarely align with the ‘fail fast, fail often’ mantra of many technology innovators. Regulators such as the National Institute for Health and Clinical Excellence (NICE) also often prefer a spread of evidence from smaller economic, usability and implementation studies. However, the sheer number of technologies available, and the pace at which they develop, also mean that regulators are limited in their capacity to evaluate the ‘tsunami’ of new digital innovation.
Effective development, evaluation and implementation often requires health and care services, innovators and technology companies to work together effectively and there are challenges here too. For the health service, this includes the need to better articulate where technology can add the most value and to spot opportunities for innovation, while for industry this includes closer working with the health service to collaboratively identify and develop suitable solutions that meet NHS needs.
But developing productive relationships between service providers and innovators can be challenging. With the NHS being a national public service, relationships between health care and industry can be complex, and issues such as intellectual property, contracting and data access can slow or even completely stall progress. High levels of system fragmentation and a complex local and national innovation landscape also mean that innovators can be unclear as to who to contact and work with.
Making sure that technology and data tools are implemented effectively and integrating them successfully into workflows are also significant challenges. It is rarely enough to simply add new technologies into existing ways of working and expect transformation. Instead, effective integration requires sufficient capacity to collaboratively redesign roles, processes and care pathways to derive the benefits of the new functionalities that technology offers – in other words, focusing on the whole change and not just the technology.
Central to effective implementation is making sure there are the necessary levels of technological skill and literacy among both staff and service users. Recent analysis shows that a fifth of the UK population do not have even the most essential digital skills, creating challenges both for the effective uptake of new technologies and for making sure that the ‘digital divide’ does not widen existing inequalities. For example, low levels of digital skills, along with poor internet access and language barriers, can mean people remain excluded from services like online appointment booking and remote appointments.
Also fundamental is building public and staff trust in the use of new technologies. After the COVID-19 pandemic hit, new technologies were rolled out with impressive speed, but Health Foundation research has shown that not everyone is yet sold on technology-enabled care. A significant minority (36%) of UK adults surveyed during the first wave of the pandemic thought that while greater use of technology made sense during the pandemic, it was not something ‘for the long term’.
We asked our survey respondents which of the technology challenges highlighted in bold above they considered to be the most significant for adopting LHS approaches; the results are shown in Box 7.
Box 7: Technology challenges – what our respondents said
The results indicate that all proposed statements were thought to be a challenge to some degree (see Figure 7), averaging a score of 3.8 out of 5 (with 5 representing ‘a very significant challenge’).
However, the highest-ranked challenge was ‘ensuring effective implementation of technology and data tools and integrating them successfully into workflows’ (4.4), followed by ‘ensuring adequate underlying technological infrastructure, such as IT hardware and software, or internet/Wi-Fi connectivity’ (4.1).
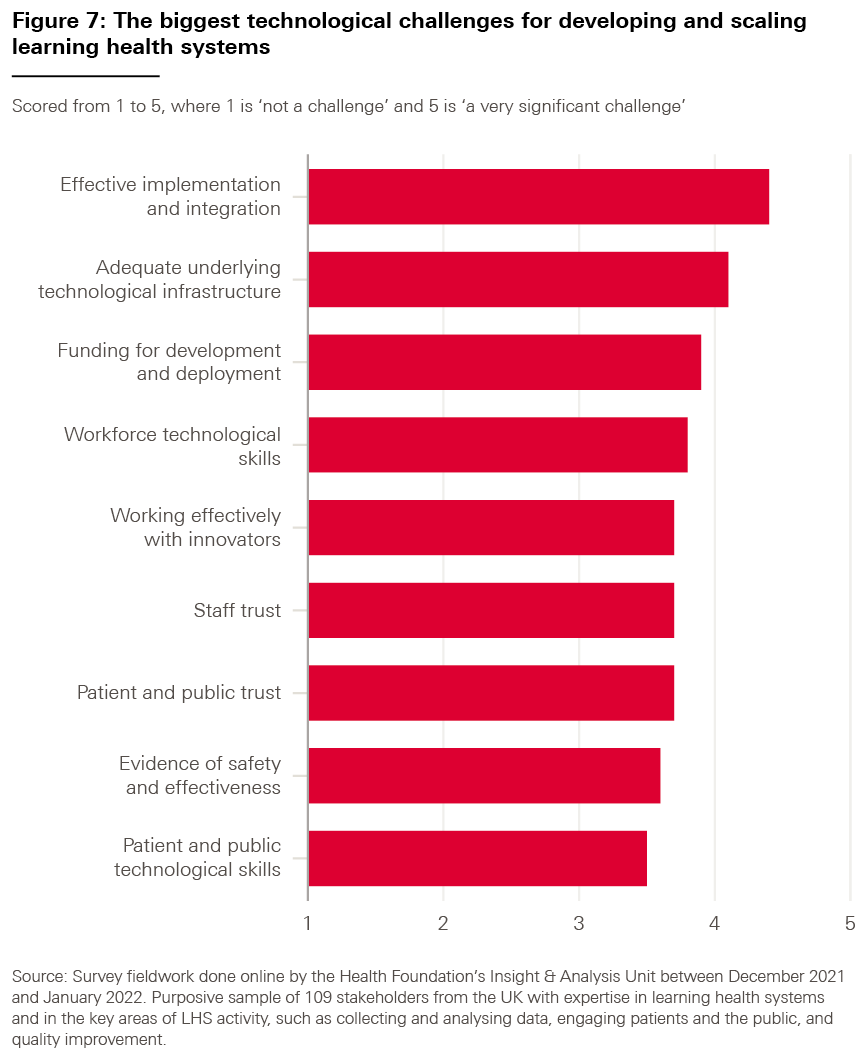
Case study 10: Reducing the health burden of diabetes with artificial intelligence-powered clinical decision tools (RADAR)
A Health Data Research UK (HDR UK) and Health Foundation Catalyst project
Nearly 5 million people in the UK have diabetes and this is predicted to rise by another 500,000 in the next decade. For people living with diabetes, effective management is critical. Uncontrolled diabetes can have serious complications and lead to heart disease, stroke and kidney failure. Diabetes costs the NHS £10bn a year, primarily due to complications like amputation and blindness. So acting early to prevent complications both improves care and quality of life and avoids complication-associated expenditure.
RADAR (Risk Algorithms for Decision Support and Adverse Outcomes Reduction) was a Better Care Catalyst project, led by North West London Health and Care Partnership, in collaboration with MyWay Digital Health, Imperial College Health Partners, AstraZeneca, the Institute of Global Health Innovation at Imperial College London and Imperial College Healthcare Trust. The aim of the project was to use data and artificial intelligence to improve clinical decision making and self-management for individuals with diabetes.
The project took models that predict the risk of complications in patients with type 2 diabetes, originally developed and validated in Scotland by MyWay Digital Health, and aimed to revalidate them using the longitudinally linked and ethnically diverse Discover dataset of more than 2.5 million people living in north-west London.
The team worked with clinicians and people with type 2 diabetes to understand whether a digital platform embedding predictive models could support self-management and enable clinicians to intervene earlier to prevent complications and hospitalisation. Specifically, the platform used algorithms to predict an individual’s risk for complications such as loss of sight, amputation and heart disease.
It included a digital interface where clinicians and people with diabetes could see their risk levels and how they compared to other members of the population. It also allowed users to see how losing weight, increasing activity or stopping smoking could reduce their risk, and work with their clinician to make changes. Users reported feeling empowered by being able to see their data, and by understanding how their actions could influence their long-term health.
Through workshops with users, the team was able to further develop the platform to make sure that it was easy to navigate and understand. In this way, the project enabled innovators to implement and further validate the risk-prediction algorithms as well as understand how artificial intelligence tools could potentially be delivered across a range of other conditions.
The RADAR project shows how emerging technologies like artificial intelligence and routinely collected data can be embedded into care pathways to support collaborative and personalised decision making. In particular, it highlights the value of having access to safe and secure population-level health data and the importance of user consultation for technological adoption and spread.
Case study 11: Cambridge University Hospitals’ eHospital programme
The eHospital programme provides a single integrated electronic patient record system for most inpatient and outpatient service information in Cambridge University Hospitals NHS Foundation Trust. Launched in 2014, the platform is accessible to trust staff, primary care colleagues and other hospitals in the region. It highlights some of the benefits that health care providers in the UK can achieve through using electronic record systems. The system is linked to the national GP Connect service, enabling clinicians to share and view primary care information.
With more than 95,000 people registered with the platform through the MyChart app, service users also benefit from greater access to health information, such as diagnoses, test results and medication history. Service users can view correspondence with clinicians, report their own data and self book appointments.
The team report that the eHospital programme has increased service user activation and empowerment and reduced the administrative and logistical burden on staff. The system also enables a range of digital services such as outpatient kiosk self-check-in, automated coding of outpatient procedures and automated letter generation, reducing the burden on administrative resources. Other significant impacts include improvements in medication safety and organisation-wide cost savings. The team has estimated that 124 nursing whole-time-equivalents are saved each year through direct recording of observations and medication administration at the bedside.
The system also supports service learning and improvement through the generation of usable real-time insights. For example, Cambridge University Hospitals NHS Foundation Trust has used insights from record data to develop alerts to support the early identification and treatment of possible sepsis.
The eHospital programme shows the importance of staff engagement and change management for successful implementation. The programme team worked with more than 1,000 hospital staff to make sure the system was configured to their needs. This included pre-launch sessions to illustrate new system workflows and gather feedback, as well as role-playing and simulations to understand how the system might interact with other processes. Patients played a critical role in configuration and deployment decisions for the MyChart platform, and the team has continuously gathered feedback, allowing for iterative improvement.
Learning from the programme highlights the need for staff education and training. For example, staff digital literacy was identified as a potential barrier to implementation, so intensive training was delivered to more than 12,000 staff before the launch. The eHospital team has also recognised the importance of building data analytics capability within the organisation, through recruiting data analysts and upskilling clinical teams in analytical methods. The team has also invested time in raising awareness about data quality and supporting staff with best practice approaches to recording information, which has led to a reduction in the need for data ‘cleaning’.
Case study 12: Project Breathe – artificial intelligence-driven clinical decision-making tools to manage cystic fibrosis
A Health Data Research UK (HDR UK) and Health Foundation Catalyst project
Cystic fibrosis, an inherited disorder affecting one in every 2,500 babies born in the UK, can cause severe damage to the lungs, digestive system and other organs. As a degenerative condition, it will get worse over a person’s lifetime and requires lifelong management. People with cystic fibrosis can also experience pulmonary exacerbations, which must be diagnosed and managed early and effectively to minimise any negative effect on their quality of life and survival.
In 2019, Project Breathe, a Better Care Catalyst project, tested whether self-reported data and data generated by home-monitoring devices could track health and wellbeing, reduce unnecessary clinic visits, better inform clinicians and improve quality of life for people living with cystic fibrosis. Collaborators from the Cambridge Centre for AI in Medicine at the University of Cambridge, Microsoft Research and social enterprise Magic Bullet (funded by the Cystic Fibrosis Trust, the National Institute for Health and Care Research and LifeArc) used these patient-owned data to develop adaptive artificial intelligence that could analyse and learn from the data in order to identify the early signs of exacerbations.
120 adults were given devices to self-monitor their lung function, activity, heart rate, oxygen saturation and weight. The pseudonymised data were stored in a secure, online system and provided to participants in real time via the Breathe app. Their clinicians also used the data to monitor early changes to health, detect signs of exacerbation and make informed decisions about when to see patients face to face and where changes to treatment might be needed.
The project has shown some early signs of impact. As a result of integrating the technology into their self-care routines, participants reported that they had developed a better understanding of their health and were able to adjust their plans or lifestyle to make sure they stayed well for longer. Almost all patients using the app have also been able to avoid unnecessary clinic attendance by remotely reviewing the data with their clinician.
The artificial intelligence analysis of the data has also predicted symptom flare-ups up to 10 days earlier than when antibiotics would typically have been given, meaning treatment can be given earlier and preventable hospitalisations and negative outcomes potentially avoided. The project has now recruited hundreds more participants from both the UK and Canada to better refine the algorithm and deepen learning.
Project Breathe is showing that, for long-term conditions such as cystic fibrosis, being able to collect and analyse up-to-date information and integrate this into decisions about care can be an effective way to empower people and support decision making. It also shows that doing so does not necessarily increase the burden on health care professionals and can simultaneously make it easier for them to monitor and manage their patients and intervene where necessary.
2.4. Nurturing learning communities
2.4.1. Key issues and opportunities
The kind of learning and improvement that LHSs undertake typically needs input from many people. This requires a community of people with different roles and backgrounds who are committed to taking part in the process (a ‘learning community’), along with an ability to bring them together, reconcile differing views and take decisions. Such learning communities can include ‘communities of practice’, improvement collaboratives and professional and patient networks. They are groups of motivated people – such as clinicians, patients, carers, researchers, analysts and improvement teams – who come together around a common goal to identify problems and collaborate on designing and implementing solutions and improvements.
Especially because of this, when thinking about and developing LHSs, it is important to not only focus on the technical aspects such as data and technology but also consider the social and relational aspects of learning and improvement. Discussions of LHSs sometimes imply that iterative learning cycles have a kind of automaticity to them – turning practice into data, data into knowledge, knowledge into practice and so on – but as our description of the learning cycle in Figure 1 (Chapter 1) illustrates, sitting at the heart of each of these stages are social processes of deliberation, problem definition, priority setting and decision making. How well people collaborate at these critical stages of the learning cycle will significantly influence how well the LHS works and how successful the resulting changes will be. The stakeholders we interviewed and the literature we reviewed highlighted how building strong learning communities makes it more likely that improvement work will lead to positive, sustainable change. For example, an evaluation of the HipQIP project (case study 15), an improvement collaborative to improve hip fracture care, found that the learning environment created by bringing people together around a common purpose played an important role in the project’s success.
Bringing diverse perspectives to the improvement process
Healthy learning communities typically have a variety of characteristics. They incorporate diverse roles and perspectives. As well as offering opportunities for professionals from a range of backgrounds to collaborate on change, they can also offer a way for patients, service users and the wider public to engage in the learning and improvement process and shape services according to what works for them. This makes it more likely that changes will achieve the desired outcomes. For example, the National Children and Young People’s Diabetes Network worked with children and families from 173 paediatric diabetes units across England who were able to shape services by informing priorities for change, contributing to improvement initiatives and accessing peer support and information. The stakeholders we interviewed also highlighted how the inclusion of diverse voices from a variety of backgrounds can challenge assumptions and help address issues of inclusion, power and paternalism in care. Here, learning communities can benefit from the involvement of voluntary and community organisations because of their ability to harness knowledge and skills in local communities, as the Health Foundation’s Common Ambition programme shows.
Effective learning communities also have trusting relationships and a culture that supports an open and honest learning environment, with the psychological safety for people to raise concerns and try out new ideas and approaches.
Mechanisms for collaboration
Learning communities also need the ability and the mechanisms to bring people together to deliberate, to aggregate or reconcile differing views and to make decisions – whether in person or online. For example, the Health Foundation’s Q Lab UK (case study 14) works with a broad group of people with interest and expertise in a particular topic, including clinicians, people with lived experience as patients, carers and service users, researchers, commissioners and national system leaders. Through collaborative research activities, such as surveys, workshops and interviews, the Lab generates intelligence and shares the findings with teams testing service changes to support improvement efforts.
Meanwhile, the growth of online research platforms such as Thiscovery (case study 13), developed by The Healthcare Improvement Studies Institute, and VOICE, led by the University of Newcastle, allows a wide range of stakeholders to take part in improvement work over the phone, on their computer or face to face.
2.4.2. Challenges
Developing and sustaining inclusive learning communities can be a significant undertaking, and our research found several challenges that can arise in the process.
First, when setting up an LHS, there may be an initial need either to create a new network or learning community or to harness existing ones. The work involved in doing so should not be underestimated. There can be a belief that learning communities are naturally occurring, where interested parties will gravitate together to learn and collaborate. But in many cases, the formation of a learning community requires considerable bespoke design, nurturing and facilitation, not least to bring together a broad range of stakeholders.
Perhaps partly as a consequence of underestimating the work involved in forming and maintaining learning communities, there can be a lack of infrastructure and funding for convening people, to enable discussion and collaboration. For example, there can be difficulties in accessing suitable physical space for face-to-face collaboration, while variation in access to suitable software and hardware can make online collaboration difficult. The stakeholders we interviewed also highlighted how securing backfill to free up clinician time for improvement (getting cover for their role while they are engaged in such work) can be exceptionally difficult, but is nevertheless highly important. This can be especially challenging when there is a desire to collaborate in large groups or beyond single teams or organisations, where many participants may need such support.
Even when the right mechanisms and resources are available, clinicians, data analysts, researchers, quality improvers and other professionals still need to find the time and make the effort to be involved in improvement work. With the health service facing chronic workforce shortages and some of the most severe pressures in its history,, this can be difficult. For LHSs operating at a large scale or over long time periods, maintaining engagement and the dynamic nature of the learning and improvement cycle can be even more challenging.
The time and effort involved can be a particular barrier for patient and public participation; without adequate financial compensation or resources, contributions are often restricted to those who can afford to take part. Some service users also face other barriers, such as ill health or difficulties with communication, which make the burden of participation even greater. Without support to participate, there is a risk that those involved in learning communities are the most motivated and engaged users, rather than a broader, more representative group.
Another challenge is that cultural barriers can make it hard for people to come together around a common set of goals. While it is important to make sure that learning communities are diverse and representative, in some cases, diversity in professional and personal backgrounds, or in organisational approaches and culture, can mean that each stakeholder sees the issues differently, making consensus hard to achieve. In cases like this, the work involved in successfully facilitating the learning community will be greater. Language matters too: when bringing groups with varied expertise together, it is important to make sure that the language used is accessible and jargon free.
Sometimes initiatives can run into resistance to change. For example, overstretched staff already grappling with multiple proposals for reform may see the work of an LHS as ‘just another initiative’ – unless the rationale for change is clearly set out and the work is aligned with broader organisational strategies. On other occasions, staff or service users may be sceptical about proposed changes, or have legitimate quality or safety concerns, especially if they have not been involved early enough in discussions or in formulating the proposals.
Another barrier highlighted through our stakeholder discussions is a lack of clarity about, or competing perceptions of, what an LHS is and what it is trying to achieve. As we discussed in Chapter 1, there is no single definition of an LHS, and discussions can be highly theoretical, making the ideas difficult to apply in the real world. There is also a wide range of models of LHSs, making it difficult for individuals, organisations and systems to decide on the best approach.
Importantly, many of the issues discussed above highlight how successful learning communities – and successful LHSs – require effective leadership. In particular, effective leadership is a critical facilitator for:
- aligning the activities of the LHS with wider operational and strategic priorities
- securing resources and organisational support
- gaining the buy-in of staff and teams across an organisation
- creating a healthy improvement culture.
In this context, effective leadership includes behaviours such as being ‘problem framers’ rather than ‘problem solvers’, listening and observing, and stimulating reflection and critical thinking within teams. (While a detailed exploration of leadership is beyond the scope of this report, it is worth emphasising that this includes clinical leadership, which can have a significant impact on organisational performance.)
We asked our respondents which of the learning community challenges highlighted in bold above they considered to be the most significant for adopting LHS approaches; the results are shown in Box 8.
Box 8: Learning community challenges – what our respondents said
As shown in Figure 8, the results indicate that all proposed statements were thought to be a challenge to some degree, averaging a score of 3.9 out of 5 (with 5 representing ‘a very significant challenge’).
However, the highest-ranked challenge was ‘the time and effort it takes to participate in the work of a learning health system’ (4.3), followed by ‘absence of the leadership needed to put in place and use learning health system approaches’ (4.1).
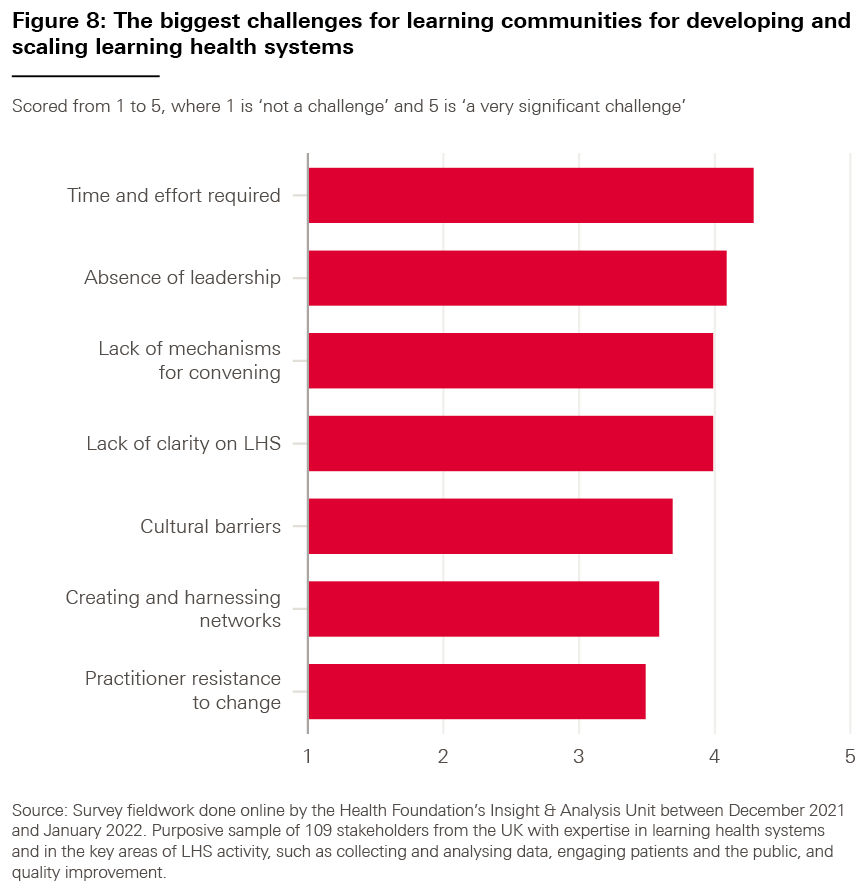
Many challenges facing health care could benefit from bringing together multiple forms of expertise to design solutions at scale. Thiscovery, an online research platform developed and hosted by The Healthcare Improvement Studies Institute (THIS Institute), helps to address this need. Founded on the principle of co-creation, it brings people who have questions about how to improve care together with people who can help build the evidence for answers – including, most importantly, patients and staff. The platform can enable a range of research, evaluation and consultation processes, including consensus-building, iterative development and the evaluation of solutions. Since April 2020, Thiscovery has been used to deliver more than 30 discrete research tasks and has more than 6,000 registered participants who can take part remotely and at different times.
One of its first projects arose in response to a problem that surfaced as the COVID-19 pandemic took hold: how to adapt clinical processes for COVID-19 scenarios. The obstetric emergency known as post-partum haemorrhage (heavy bleeding after birth) was one important case where new processes were needed to keep people safe and still deliver high quality care and experience. Having separate units devising processes in silos is time-consuming and wasteful, but it also wasn’t going to be possible to use the in-person meetings characteristic of pre-pandemic collaborative approaches.
A novel approach was required to enable rapid iterative learning at scale while minimising participant time and effort. Thiscovery enabled this with more than 100 experts in maternity care taking part through a novel consensus-building methodology to generate recommendations, with participants given rapid feedback via interactive charts. The project produced rapid consensus on 16 practical, actionable recommendations to adapt usual care for COVID-19. Major professional bodies and NHS services endorsed the results, including a video showing the adapted processes. The project continues to contribute to education, with more than 130,000 views of the online video, and the approach used has been codified into a formal methodology, which is already being used as the basis of other projects.
Recent projects show how Thiscovery can support learning communities and play a key role in LHSs by offering a mechanism to understand problems, co-create visions and evaluate solutions. The co-design approach is especially important in making sure that solutions can be developed and tested at scale in ways that allow diverse priorities, views and ideas to be respected, while the quality of engagement, ownership and endorsement increases the chance of impact.
Q is a community of thousands of people across the UK and Ireland, collaborating to improve the safety and quality of health and care. Members share their knowledge and support each other to tackle challenges, enabling faster progress in improving health and care.
Q supports a network of labs across the UK and Ireland. The network currently includes Q Lab UK and Q Lab Cymru. Q Lab UK uses creative and collaborative approaches to work on a single complex challenge and brings together organisations and individuals from across the UK and Ireland to pool knowledge about the topic. They then uncover insights and develop, test and scale ideas. The Lab also draws on approaches and tools from quality improvement and disciplines such as social innovation and design.
Since 2017, Q Lab UK has explored themes such as the importance of peer support and improving care for people living with both mental health problems and persistent back and neck pain. The Lab is currently working in partnership with NHS England on how to build staff and patient trust and confidence in technology-enabled remote monitoring, so that it can be scaled across the health and care system.
To explore an issue, the Lab works with a broad group of people with relevant interest and expertise from a range of backgrounds both within and outside of the health and care sectors. The Lab instigates collaborative research activities, such as surveys, workshops and interviews, to generate initial intelligence. The Lab then invites leaders within health care organisations to apply to become ‘test teams’. These teams are given tailored coaching and support to work together to identify potential solutions, and test and scale them over time.
The test teams, along with a range of contributors from the Q community, convene regularly through participatory workshops, where they learn and apply methods for design thinking and collaborative working. The learning and insights collected from the Lab community are shared widely and used to support improvement efforts in their own work and beyond.
A recent evaluation of Q Lab UK shows that teams appreciate the ways of thinking and working used in the Lab, particularly around participation and collaboration. People also value the opportunity and encouragement to be curious, to slow down and to ask bold questions. They also find the process a good opportunity to be exposed to methods that help better understand service users’ needs and to have the space to practise creative facilitation skills.
It is through Q Lab UK’s convening infrastructure and processes that participants develop new ideas, skills and capabilities. Importantly, they also work together as a learning community to build shared purpose, supportive relationships and collective energy, which helps to create the context and conditions for scaled impact in health and care.
2.5. Implementing improvements to services
2.5.1. Key issues and opportunities
While gathering data and drawing knowledge and insights from them are key components of any LHS, the goal is to put this into practice and make improvements to care quality and safety. This involves understanding how to identify, design and implement changes to service delivery, and then evaluating the impact of those changes to see whether they have had the desired effect.
Quality improvement skills and methods
Quality improvement – the systematic and coordinated use of specific methods and tools, with the aim of bringing about a measurable improvement in quality – can play an important role in supporting the design and implementation of service changes. Quality improvement tools include ‘driver diagrams’ to understand cause and effect, and methods for improvement such as ‘Lean’ principles or experience-based co-design. Quality improvement approaches sit well with LHSs as they are similarly based on an iterative approach to testing – for example through ‘plan–do–study–act’ cycles.
Training and coaching can help develop the use of quality improvement in practice and maximise its value. For instance, PINCER (case study 2), a pharmacist-led intervention to identify potentially harmful prescribing in general practice, teaches pharmacists how to use quality improvement tools and gives them the opportunity to apply these tools in general practices to help reduce prescribing errors. It includes action learning sets for pharmacists to discuss their experience with their peers and get advice on how to hone their quality improvement skills.
Beyond developing improvement skills, there are a range of other important ways in which the implementation of service changes requires support, which have been discussed in previous sections, such as providing clinical backfill or providing assistance with data analytics and evaluation.
Taking an organisation- or system-wide approach to improvement
Quality improvement tools and methods are not enough in themselves to drive improvement, however. A positive workplace culture that is conducive to improvement also matters, as does the presence of an integrated strategy for improvement at the organisation or system level. It can be difficult to implement and sustain improvements if they are not aligned to wider organisational or system priorities, or in the absence of visible, long-term support from senior leaders who are willing to give front-line teams the space and permission to do things differently.
The relationship between quality improvement and quality planning, control and assurance is also relevant here. Until recently, many organisations have treated these four elements of the ‘quality management system’ as separate entities, each with their own governance processes and strategic goals – a practice that has led to disconnected and sometimes conflicting aims and activities. This is now beginning to change. For example, organisations such as East London NHS Foundation Trust are choosing to take a single approach to quality that encompasses the full quality management cycle. The Trust’s approach is based on engaging staff and the local community at the planning stage to identify where improvement work should be targeted over a five-year period, using quality improvement methods to implement changes, and then making sure improvements are maintained.
Learning across boundaries and improving collaboratively
Learning from peers – particularly those who are facing the same challenges – can be an effective way to build solutions for improving health care services and address unwarranted variation, for example through models such as breakthrough collaboratives and action learning sets. For example, HipQIP (case study 15), an initiative to improve outcomes for people with hip fractures, shows how the collaborative model can drive improvement. Teams from across the UK collaborated to design changes to hip fracture care, test them in their respective organisations and share their results, supported by guidance in quality improvement. But while these kinds of models can be effective, their success depends on the context they are deployed in and whether they are sufficiently resourced.
2.5.2. Challenges
Our experience of funding innovation and improvement projects at the Health Foundation over the past two decades has shown that implementing change in health care services can be a challenging and complex endeavour, requiring specific capabilities and resources.
One key challenge can be a lack of skills and knowledge required to effectively implement improvements to care. Health Foundation research has found that these capabilities are essential for high-performing health care organisations, and they were recognised in the NHS Long Term Plan as an important area for development., A related challenge is how quality improvement is taught to staff.,, Sometimes the focus is primarily on the technical aspects of improvement, neglecting important relational aspects, such as how to build a team, motivate others and secure support. And some quality improvement programmes do not give staff enough opportunities to implement their skills.
Also a challenge is a lack of capacity – the time and space needed for people to effectively implement improvements to care. The capacity required to plan, set up, execute, evaluate and refine service changes is often underestimated and can be very difficult to find when services are understaffed and facing significant demand pressures. And both building capability and creating capacity require resourcing, so a lack of financial and other support to effectively implement improvements to care is another related challenge.
Earlier on we highlighted how the culture of an organisation – the shared ways of thinking, feeling and behaving – is a critical ingredient for an effective LHS. The experience of NHS Nightingale Hospital London (case study 4), which was set up temporarily to create sufficient intensive care capacity to treat patients with COVID-19, shows how an effective LHS relies on the right culture and behaviours among staff at every level – not only among senior leaders. As Nelson and others put it, an LHS is more than simply ‘technical infrastructure’; strong values and a commitment to collective learning need to govern it. Consequently, the absence of a culture of learning and improvement within health and care services can be a barrier to developing LHSs. Research suggests that the prevailing culture in some services is too often defensive, inward looking and hierarchical – characteristics that not only inhibit the improvement of quality but also can have an adverse impact on it.,,
Given the relatively underdeveloped nature of LHSs in the UK, it is fair to say that, while there are many successful examples of improvement going on, few of them take place within an LHS. For instance, they may be time-limited projects or projects not necessarily taking a systematic approach to analysing and drawing knowledge from data. And where actions to improve service delivery do occur within an LHS, there may not be explicit acknowledgement that an LHS approach is being taken. Indeed, many of the teams we interviewed for this report whose work exemplified LHSs were not aware that their projects were taking LHS approaches. For these reasons, while this report documents a series of case studies, there remains a lack of evidence or practical examples of successful LHSs, showing what impact they have had and why. Other issues contribute to this evidence gap too. It is often the case that improvements are incremental and accrue over time. The stakeholders we interviewed told us that there can also be a reluctance to learn from failure, so where attempts to build LHSs have failed, they are less likely to be reported. In addition, our interviewees also indicated that traditional evaluation models such as randomised control trials do not lend themselves to LHSs.
Another challenge for implementing improvements to services is the gap between health care research and practice. Research can provide a critical input into the work of an LHS, and an important objective will be to quickly translate research findings into improvements in practice. But research does not always have a clear practical application and the results do not always reach health care professionals. Even when they do, significant work may be required to interpret and implement the findings. Our case study of PReCePT2 (case study 16), which aims to increase the uptake of magnesium sulphate to reduce brain injury in preterm babies, shows that even though there was compelling evidence to support the use of magnesium sulphate, it took several years of systematic quality improvement work and national support to embed it into widespread practice. Thiscovery (case study 13), an online collaborative research platform developed by The Healthcare Improvement Studies Institute, shows one way in which this gap can be bridged – by bringing together researchers and practitioners and making sure that research projects are designed to have a practical application from the outset.
We asked our survey respondents which of the improvement challenges highlighted in bold above they considered to be the most significant for adopting LHS approaches; the results are shown in Box 9.
Box 9: Improvement challenges – what our respondents said
As shown in Figure 9, the results indicate that all proposed statements were thought to be a challenge to some degree, averaging a score of 4 out of 5 (with 5 representing ‘a very significant challenge’).
However, the highest-ranked challenge was a ‘lack of capacity (time, space and so on) for people to effectively implement improvements to care’ (4.6), followed by ‘lack of financial and other support’ (4.0) and ‘the gap between health care research and practice, which hinders the translation of research into practice’ (4.0).
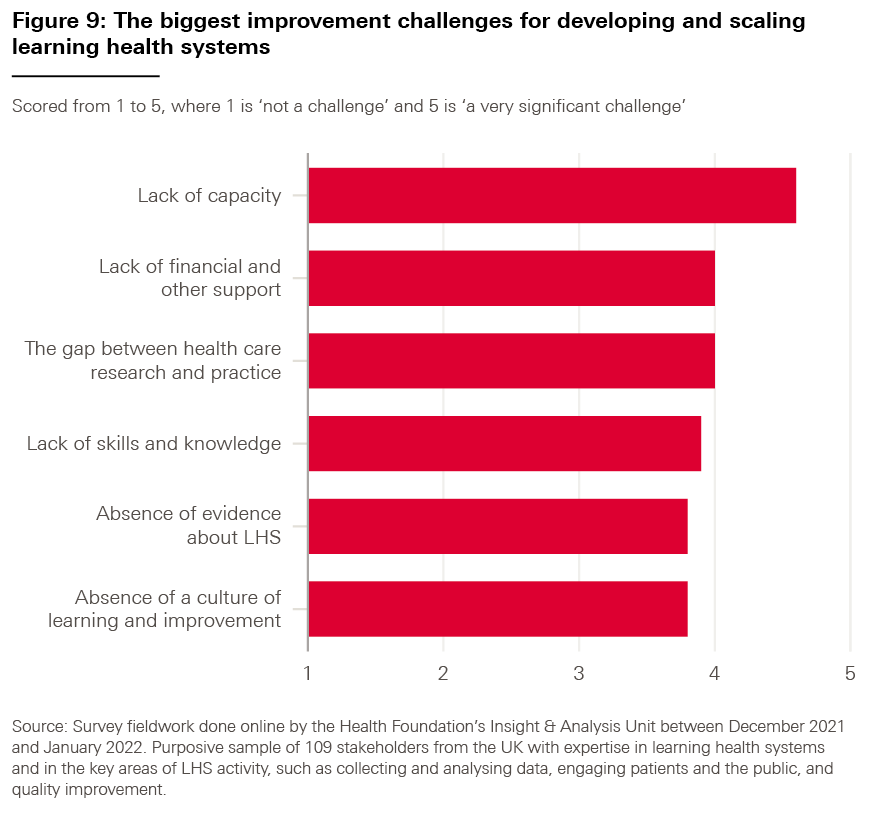
Case study 15: HipQIP – hip fracture quality improvement programme
Hip fracture is the most common serious injury in older people and costs the NHS and social care more than £1bn a year. Nearly a third of people who fracture their hip will die within a year and a fifth of patients will not return to their own home. Northumbria Healthcare NHS Foundation Trust leads the hip fracture quality improvement programme (HipQIP), which aims to improve care and outcomes for people presenting to hospital with a fragility hip fracture (one that results from minimal trauma, such as a slip or fall). Initially this was developed for use within Northumbria but it has been scaled successfully to five acute hospital trusts – four in England and one in Scotland.
The programme focuses on prioritising additional nutrition for patients with hip fractures; implementing a surgical care bundle, pain block in A&E and surgery within 36 hours; and carrying out root cause analysis of any deaths; all while gathering outcome data and learning from different sites on their approaches to improve care.
Independent peer reviews of each department allowed for accurate baselines before interventions. Teams then attended learning events to review evidence and share ideas about improvement solutions. They tested and implemented iterative changes in their local settings and collected data to measure the impact of the work. This approach helped to build a strong ‘breakthrough collaborative’ – a learning community across the five different trusts that offered a safe space for both raising concerns and sharing learning between individuals and organisations.
In an 8-month period, the four English hospitals recorded 119 fewer deaths than expected if mortality had remained at the baseline and 77 fewer deaths than at the hospitals used as controls during the same period. 30-day mortality rates in hospitals within the collaborative reduced from 9.2% to 5.8%. Furthermore, 100 extra patients returned home instead of going to a nursing or residential home, and length of hospital stay was reduced by 2 days compared with the baseline.
In successfully scaling to five additional trusts, HipQIP shows the importance of working with peers to share learning and spread good practice, which can facilitate more robust improvement. Independent peer evaluations provided the necessary baseline to help trusts objectively understand where they started from and where to focus their improvement efforts. The programme also shows the importance of evidence-based quality improvement approaches, such as the Institute for Healthcare Improvement’s Collaborative Model, which enabled providers to confidently identify areas of good practice and areas requiring further iterative improvement.
Case study 16: Reducing brain injury through improving uptake of magnesium sulphate in preterm deliveries (PReCePT2)
Around 60,000 babies are born prematurely in the UK each year. These preterm babies are at greater risk of brain injury and conditions such as cerebral palsy, which affects 500 premature babies a year. Evidence shows that mothers at risk of premature delivery who take magnesium sulphate during preterm labour and before birth can reduce the risk of their babies developing cerebral palsy by a third. The cost of a dose is less than £1, but less than half of eligible mothers were receiving the treatment in 2016.
From 2014 to 2015, the PReCePT1 project was implemented in five West of England maternity units. During the project, maternity units and mothers co-developed a Quality Improvement Toolkit, which provided practical tools and training to increase knowledge and awareness of using magnesium sulphate to protect against preterm brain injury. As a result, the number of women receiving the preventative treatment in these units increased by an average of 25%.
Following this success, the project was supported to scale up nationally, called PReCePT2. From 2017 to 2020, 13 maternity units received an enhanced quality improvement intervention in addition to the standard model of implementation, including bespoke one-to-one and team coaching and access to learning events. ‘Snapshot’ tools, such as communication and implementation plans, were developed so that staff could understand how much progress they had made, alongside tools to plan how changes would be sustained.
The project shows how using data to evidence the need for improvement and sharing service users’ stories to highlight impact can be effective ways of gaining buy-in. PReCePT2 uses performance data to stimulate action, including data on the number of people not getting magnesium sulphate, cases that could be avoided and the cost comparison between the intervention and cases of cerebral palsy.
PReCePT2 also highlights the importance of bringing together all professionals involved in a care pathway to collaborate on improvement, and of planning for long-term sustainability at the outset. PReCePT has now been adopted by all 152 maternity units in England and magnesium sulphate uptake has increased to more than 85% for eligible mothers. As a result, it is estimated that 48 cases of cerebral palsy were prevented between 2018 and 2021, saving £38.4m in lifetime health and social care costs.
Making learning health systems a reality
3.1. What actions can policymakers and organisational and system leaders take?
Action is needed across all four of the areas discussed in Chapter 2 – data, technology, learning communities and improvement – to support the development and spread of learning health systems (LHSs) in the UK. Not only is each of these areas important in its own right for driving improvement in health and care, beyond the role it can play as a component of LHSs, but, as discussed in Chapter 1, we think that the diversity and complexity of LHSs mean that the most practical way to make progress on this agenda is by focusing on developing and improving the activities and assets that underpin them.
We used our survey of expert stakeholders to understand what actions policymakers and organisational and system leaders should prioritise. This chapter sets out what our respondents told us were the highest-priority actions and offers recommendations for how they could be taken forward.
3.1.1. Priority actions for policymakers
For each of the four areas explored in Chapter 2, we gave our survey respondents a set of potential actions for policymakers (including government ministers, civil servants and leaders of public bodies). We asked them to tell us how much of a priority they thought these actions should be, specifically for supporting the development of LHSs, ranging from ‘low priority’ to ‘very high priority’. (We also asked our respondents to tell us whether there were any additional policy actions we might have missed in our survey, but responses suggested that there were no significant omissions.)
Table 3 shows the top three policy priorities chosen for each of the four areas, and how they rank based on the number of the people who told us they were a ‘very high priority’.
Table 3: Top three policy actions by key area, ranked
|
Rank |
Area |
Policy action |
% selecting ‘very high priority’ |
|
1 |
Technology |
Provide more funding and support to improve digital maturity and help create the infrastructure needed for LHSs |
68% |
|
2 |
Improvement |
Provide more funding and support for building capability to implement improvements to care |
63% |
|
3 |
Learning communities |
Develop a clear narrative about why LHSs matter and the impact they could have |
59% |
|
4 |
Data |
Provide more funding and support to build specialist analytical and data science capability in the health and care workforce |
57% |
|
5 |
Data |
Provide more funding and support to help providers meet interoperability standards |
51% |
|
6 |
Technology |
Provide more funding and support for the evaluation of technology in real-world settings |
48% |
|
7 |
Data |
Improve regulation and standards to enable effective data sharing while protecting privacy and reassuring patients and staff |
47% |
|
8 |
Technology |
Provide more funding and support for the implementation of technology |
47% |
|
9 |
Improvement |
Provide incentives to encourage and reward the implementation of improvements to care |
44% |
|
10 |
Learning communities |
Make sure learning communities are inclusive by providing funding and support that address social, cultural and economic barriers to participation |
43% |
|
11 |
Improvement |
Provide more funding and support for the evaluation of LHSs to build the evidence base |
43% |
|
12 |
Learning communities |
Provide more support for patient and community groups to voice service user perspectives in health and care improvement |
42% |
Without diminishing the importance of action on all of these 12 issues, and indeed all of the issues covered in our survey, for our policy recommendations in this report we have chosen to focus on the five actions that more than half of our survey respondents told us should be a ‘very high priority’. These include actions from each key area within our report – learning from data, harnessing technology, nurturing learning communities and implementing improvements to services – highlighting the need for action across all of these areas.
We now set out these highest-ranked priorities – starting with the need for a clear narrative, before moving on to digital maturity, data, interoperability and improvement capability – and make recommendations for specific steps that policymakers could take to advance them.
1. Develop a clear narrative about why LHSs matter and the impact they could have.
Key actions that can be taken here include:
- Formulate a clear vision and set of principles for developing and adopting LHS approaches, which set out the value of LHSs and how they can be developed at different levels of the system. This should include a clearer articulation of the potential role of LHSs in wider service transformation plans. The narrative set out in this report aims to contribute to this wider vision.
- Alongside research funders – for example, UK Research and Innovation (UKRI) and, in England, NHS England and the National Institute for Health and Care Research (NIHR) – help to build the evidence base for LHSs, for example through commissioning a series of evaluations to better understand how LHS methodologies have contributed to improved outcomes and to identify barriers to the adoption of LHS approaches.
- Make sure that there is better alignment of policies across health care improvement, digital and data, leadership development and wider system transformation. Action here should seek to increase the use of LHS approaches and support their ability to deliver on broader policy agendas (for example, delivering a net zero NHS or reducing health inequalities).
- Encourage and support clinical, improvement and data communities – for example, the Q community, the Better Care community and other professional networks – to enable the sharing of learning and expertise across LHSs.
2. Provide more funding and support to improve digital maturity and help create the infrastructure needed for LHSs.
Key actions that can be taken here include:
- Direct more consistent, longer term funding and support to the least digitally mature health and social care providers (for example, by building on the Digital Aspirant programme in England). This should include making sure that existing IT platforms can provide the necessary functionality, and also supporting the introduction of new systems and technologies where these are required.
- Related to the above, expand the availability of best practice guidance for identifying, procuring and embedding digital tools and infrastructure, including electronic health records and clinical decision support systems.
- Make sure that workforce digital skills and knowledge form an integral part of organisational and system-level digital maturity assessments.
- Building on the learning from Health Data Research UK’s (HDR UK’s) Better Care programme, support the development and spread of new analytics and data tools that can help patients, clinicians, organisations and systems to learn and improve care – for example, clinical decision support systems and risk prediction tools. HDR UK’s ‘Better Care Loop’ (see Box 10 below) sets out a vision of how this can be achieved.
3. Provide more funding and support to build specialist analytical and data science capability in the health and care workforce.
Key actions that can be taken here include:
- In line with the recommendations of the Goldacre review and the Data Saves Lives strategy, support the professionalisation of the data analytics workforce, working with the Faculty of Clinical Informatics, the Association of Professional Healthcare Analysts (AphA) and others. This includes standardising roles, training and career progression, as well as championing the use of data analytics skills within multidisciplinary health care teams. Alongside this, NHS England should secure the long-term futures of communities of practice such as AphA and NHS-R, which can support collaboration between analysts.
- Alongside data communities (for example, HDR UK, AphA and NHS-R), promote the availability of open-source data tools for all analysts to use.
- Relevant national bodies, such as Health Education England and the NHS Wales Modelling Collaborative, should develop training programmes to support the wider health care workforce to gain skills and knowledge in data science and analytics.
4. Provide more funding and support to help providers meet interoperability standards.
Key actions that can be taken here include:
- While interoperability has been a longstanding ambition, progress towards it has been slow. Policymakers need to understand why this is the case and what the deliverability challenges are. They also need to make sure that the lessons learned are brought to bear on tackling barriers and driving further progress on interoperability.
- Building on this knowledge, as well as on the NHS Interoperability Framework, accelerate progress on developing and meeting interoperability standards.
- Given that interoperability is difficult and expensive to achieve, give guidance on where it will add most value and where other ways of enabling access to information might be more suitable.
5. Provide more funding and support for building capability to implement improvements to care.
Key actions that can be taken here include:
- Develop a framework to help providers and systems (including integrated care systems in England) assess their readiness to apply LHS approaches and their capability to implement improvements.
- Given the financial pressures that local systems are under, and the upfront investment required to build improvement capability, policymakers should make seed funding available for providers and systems so that they can begin to build their improvement capability, while supporting them to find sustainable ways to fund this over the long term.
- Consider what implementation support may be required for providers and local systems within centrally led service transformation initiatives, which will help them to use LHS approaches to support the embedding, adaptation and refinement of new service models.
- Building on the learning from HDR UK’s Better Care programme, encourage the development of training modules for health care professionals on how LHS approaches can be taken, and online repositories of knowledge and tools that can support the implementation of LHS approaches.
- Support the collation and promotion of tools that are available to help people who are trying to develop LHSs, such as the LHS Toolkit.
Box 10: The HDR UK Better Care Loop – developing tools to improve decision making
Developed by HDR UK for the Better Care programme, the Better Care Loop, shown in Figure 10, visualises how data analytics and tools can be developed, deployed and spread to improve decision making in health care.
3.1.2. Priority actions for organisational and system leaders
We also presented our survey respondents with a list of possible actions for organisational and system leaders (those in leadership roles in providers and local and regional health care systems) to support the development of LHSs, and asked them to tell us what the highest priorities were. Table 4 shows the percentage of respondents who told us that each action should be a ‘very high priority’.
Table 4: Priority actions for organisational and system leaders, ranked
|
Rank |
Organisational action |
% selecting ‘very high priority’ |
|
1 |
Foster a culture of continuous learning and improvement among staff at all levels of the organisation |
70% |
|
2 |
Support front-line teams to implement improvements to care as part of LHS work |
68% |
|
3 |
Invest in the improvement capabilities required for a successful LHS |
67% |
|
4 |
Make sure there is protected time for staff to work on improvement projects |
60% |
|
5 |
Invest in the technological and data capabilities required for a successful LHS |
59% |
|
6 |
Develop a strategy to create LHSs that meet the needs of the organisation |
58% |
|
7 |
Make sure that there are roles at senior level with a specific responsibility for continuous learning and improvement – for example, chief analytical officers and chief improvement officers |
58% |
|
8 |
Bring together people who are involved in research and analysis with those involved in health care delivery and improvement |
45% |
|
9 |
Secure support from staff for adopting LHS approaches |
41% |
|
10 |
Put in place mechanisms to evaluate LHS approaches |
34% |
While most of the actions were seen as a ‘very high priority’ by a majority of respondents, there were three in particular that stood out above the pack, with more than two-thirds of respondents saying these were a ‘very high priority’. We have chosen to target our recommendations on these three actions, as listed below.
6. Foster a culture of continuous learning and improvement among staff at all levels of the organisation.
Key actions that can be taken here include:
- Health care providers and systems should make sure there is board-level responsibility for learning and improvement, as well as research and innovation. This could be through roles such as the chief quality officer role, which some NHS trusts have created, or through other approaches.
- Health care providers and systems should see learning and improvement as a central part of their organisational strategy and make sure that learning and improvement work is aligned with wider organisational and system goals.
- Organisational and system leaders have a critical role to play in fostering a healthy learning and improvement culture – in particular, by creating an environment where staff feel they are able to raise problems and have permission to test new ways of working. They can also play a valuable role in articulating what LHSs are and how they can support improvements to services.
7. Support front-line teams to implement improvements to care as part of LHS work.
Key actions that can be taken here include:
- As resources allow, organisational leaders should enable protected time for clinical and managerial staff to take part in the work of LHSs and engage in learning communities.
- Staff should be able to access training and coaching from experienced improvement practitioners and they should be given the opportunity to apply their skills in practice. The ‘dosing’ of training and skills required for different staff members will vary according to their level of involvement in improvement.
8. Invest in the improvement capabilities required for a successful LHS.
Key actions that can be taken here include:
- Organisations should make sure they have access to the relevant in-house expertise needed to use LHS approaches, in particular in data, technology, research and improvement.
- Organisations should develop the skills, space and infrastructure needed to effectively convene multidisciplinary, inclusive learning communities to design improvements together.
The above recommendations span a range of issues. But they all underscore the importance of effective leadership if we are to realise the potential of LHSs. This was a recurring theme in our interviews with stakeholders, who highlighted that effective leadership at all levels (organisational, clinical, operational and so on) will be a critical factor for the success of LHSs.
While our recommendations are aimed at showing how policymakers and organisational and system leaders might create the right conditions for the development of LHSs, our research also highlighted some practical considerations for those putting LHS approaches in place, which we set out in Box 11.
Box 11: How to get started in developing a learning health system
The analysis above suggests some important steps that will need to be taken when developing an LHS approach.
- Identify areas of health care where there is a need for improvement and establish what work is already underway to address this, and which stakeholders are involved. Where possible, it will be important to identify and build on the energy and motivation that already exists. It is also important to consider at what level to address the problem (for example, within a provider, across a group of providers or across a large national network).
- Convene stakeholders to form a learning community. Given learning communities drive the work of an LHS, this should be done at an early stage, and include a diverse mix of people – including service users and those directly involved in service delivery.
- Develop an improvement ambition. Through discussion and deliberation, the learning community should agree collectively what improvements they want to bring about and which issues to prioritise. The improvement ambition may evolve and change over time, but is important for guiding the LHS’s work and uniting people around a common goal.
- Make sure there is senior-level support. An LHS requires resources and should align with the wider strategic aims of the organisation or system in which it operates. Senior buy-in, including board-level sponsorship, is essential for smoothing the ground. Here, it may be important to emphasise how LHS approaches can embed improvement work into ‘business as usual’, and may in many cases offer a more systematic approach for taking forward existing improvement work.
- Identify and map the assets and relevant improvement activity already in place. As we set out in Figure 4 (Chapter 1), LHSs bring together a range of assets and activities. It is therefore important to identify which of these exist already, and how they could form the basis of an LHS.
- Identify the assets that do not exist or need further development and set out steps for improving them. It is important to analyse what capabilities and infrastructure require development, and plan how to achieve this.
- Select an initial goal to target within the overarching improvement ambition. While the overall improvement ambition might be long term, it is important to set a nearer term goal, to give the learning community something realistic to aim for.
- Plan the data collection and improvement activities required to achieve the improvement goal. After completing the steps above, it should be possible to identify the first set of practical steps to initiate the learning and improvement cycle.
Conclusion
This report has explored the opportunities and challenges for developing learning health systems (LHSs) in the UK, and highlighted priority actions for policymakers and organisational and system leaders. These include the need for a clear narrative about LHSs and how they can help – to galvanise progress and help those working in health and care to understand how they can engage with this agenda – to which this report aims to contribute. They also include developing many aspects of digital, data and improvement capability as well as steps to foster a culture of learning and improvement at organisational or local system level. And they include actions to nurture learning communities, to bring together diverse perspectives and bridge the divide between research and service delivery, as well as mechanisms to share learning and expertise across LHSs.
In addition to our recommendations for action, a range of other important messages about LHSs have emerged from our research. First, LHSs are not a separate agenda from the day-to-day business of service delivery; on the contrary, they are powerful precisely because they align improvement work with the process of delivering services. Nor is the LHS agenda necessarily about catalysing an ever-increasing number of new improvement projects: learning and improvement are already happening in most provider organisations and, in many cases, LHS approaches can offer a more systematic way to undertake this existing work.
As our case studies illustrate, there are many different types of LHS. And while there are vocal advocates for particular models, in this report we have aimed to highlight what they all have in common and suggested that it is by focusing on these common activities and assets that progress on the LHS agenda can be made. This ‘disaggregated’ approach (focusing on the underpinning activities and assets) also appeals because developing good data analytics, patient engagement and quality improvement are all important objectives in their own right, beyond the role these things can play as components of a full LHS. Looking at things in this way can also help people identify the seeds of LHSs in their current practice. And for organisations, systems and communities that do want to develop full LHSs, the first steps will often be to build these component parts. LHSs are not ‘all or nothing’ in this respect and are as much about an ongoing journey as any particular destination. Nevertheless, it is ultimately only by bringing these components together that it is possible to capitalise on the dynamic improvement potential of a full LHS.
We have seen that the growth in the availability of data and the increasing sophistication of technologies such as artificial intelligence present very significant opportunities for creating new digital tools to support both service delivery and improvement – for example, by linking electronic health records with clinical decision support systems. But alongside the technical side of LHSs, our research also highlights the importance of the human side. LHSs are social phenomena, relying on diverse stakeholders coming together to identify problems, design solutions and help drive change. We heard that there are important opportunities for LHSs to bring data and quality improvement communities closer together, and to make sure there is greater involvement of patients and the public in improvement. But an important message from our stakeholder engagement is not to underestimate the effort and design work involved in creating or harnessing networks to support service improvement.
Finally, the report has also explored some of the opportunities for deploying LHSs. We heard that LHSs will have an important role to play over the coming years in supporting service transformation and driving change across multiple local providers. For example, in England there is a significant opportunity now to develop these capabilities within integrated care systems – as a way of supporting the implementation, adaptation and refinement of some of the new models of care that NHS England has identified as priorities.
Beyond this, LHSs also have a critical role to play in enabling locally led service change. Ultimately, giving providers the capability to diagnose and solve problems in a robust and systematic way will need to be part of any sustainable, long-term strategy to meet the challenges that health and care services currently face.
Acknowledgements
The authors would like to thank a number of people who have provided helpful guidance, comments and insights during the production of this report. These include: Simon Ball, Ellen Coughlan, George Delal, Alistair Denniston, Jennifer Dixon, Tom Foley, Malte Gerhold, Sarah Henderson, Matt Hill, Bryan Jones, Josh Keith, Philip Scott, Adam Steventon, Paul Sullivan, Charles Tallack, Alice Turnbull, Will Warburton and Jeremy Wyatt.
We would also like to thank all those who shared their time and expertise with us in the development of our case studies. These include: Ashley Akbari, Janet Allen, Dominique Allwood, Mark Biddle, Anna Billington, Afzal Chaudry, Ruth Cousens, Carol Dezateux, Mary Dixon-Woods, Tom Downes, Rebecca Fisher, Andres Floto, Georgina Francey, Charles Haworth, Harry Hemingway, Alvina Lai, Karen Luyt, Emma Mordaunt, Jen Morgan, Mome Mukherjee, Emma Perkins, Mike Reed, Chris Roberts, John Robson, Sarah Rodgers, Joanne Scott, Jenny Shand, Simon Thomson, Debbie Wake, Martin Wildman, Bryan Williams, Ingrid Wolfe and Sophie Young.
When referencing this publication please use the following URL: https://doi.org/10.37829/HF-2022-I06
References
- HDR UK. Better Care [webpage]. HDR UK; no date (www.hdruk.ac.uk/research/our-scientific-priorities/better-care).
- Health Foundation. Catalyst projects [webpage]. Health Foundation; no date (www.health.org.uk/funding-and-partnerships/programmes/catalyst-projects).
- HDR UK. Moving the learning health agenda forward through insight for policy and practice [webpage]. HDR UK; no date (www.hdruk.ac.uk/projects/moving-the-learning-health-agenda-forward-through-insight-for-policy-and-practice).
- Ferris T, Whitty M, Dark B. Update on Life Sciences: Future of Life Sciences in the NHS. NHS England and NHS Improvement; 2022 (www.england.nhs.uk/wp-content/uploads/2022/03/BM2210Pu-future-of-life-sciences-update-march-2022.pdf).
- Institute of Medicine (US) Roundtable on Evidence-Based Evidence; Olsen LA, Aisner D, McGinnis JM (eds). The Learning Healthcare System: Workshop Summary. National Academies Press; 2007 (www.ncbi.nlm.nih.gov/books/NBK53494).
- Q. community [webpage]. Q; no date (https://q.health.org.uk).
- NHS-R Community. Welcome to NHS-R Community [webpage]. NHS-R Community; no date (https://nhsrcommunity.com).
- Foley T, Horwitz L, Zahran R. Realising the Potential of Learning Health Systems. The Learning Healthcare Project; 2021 (https://learninghealthcareproject.org/wp-content/uploads/2021/05/LHS2021report.pdf).
- Scobie S, Castle-Clarke S. What Can the NHS Learn from Learning Health Systems? Nuffield Trust; 2019.
- Nelson EC, Dixon-Woods M, Batalden PB, Homa K, Van Citters AD et al. Patient focused registries can improve health, care, and science. BMJ. 2016; 354: i3319.
- Friedman CP, Rubin JC, Sullivan KJ. Toward an information infrastructure for global health improvement. Yearbook of Medical Informatics. 2017; 26(1): 16–23.
- Wouters RH, van der Graaf R, Voest EE, Bredenoord AL. Learning health care systems: Highly needed but challenging. Learning Health Systems. 2020; 4(3): e10211.
- Spear S. Fast Discovery: The Imperative for High Velocity Learning by Everyone, About Everything, All of the Time. Health Foundation; 2017 (www.health.org.uk/publications/fast-discovery).
- Allcock C, Dormon F, Taunt R, Dixon J. Constructive Comfort: Accelerating Change in the NHS. Health Foundation; 2015 (www.health.org.uk/publications/constructive-comfort-accelerating-change-in-the-nhs).
- Ham C. Reforming the NHS from Within: Beyond Hierarchy, Inspection and Markets. The King’s Fund; 2014 (www.kingsfund.org.uk/publications/reforming-nhs-within).
- Berwick D. A Promise to Learn, a Commitment to Act: Improving the Safety of Patients in England. gov.uk; 2013 (www.gov.uk/government/publications/berwick-review-into-patient-safety).
- Hunt J. Making healthcare more human-centred and not system-centred [speech]. Department of Health and Social Care; 2015 (www.gov.uk/government/speeches/making-healthcare-more-human-centred-and-not-system-centred).
- Department of Health and Social Care. Integration and Innovation: Working Together to Improve Health and Social Care for All. DHSC; 2021 (www.gov.uk/government/publications/working-together-to-improve-health-and-social-care-for-all/integration-and-innovation-working-together-to-improve-health-and-social-care-for-all-html-version).
- NHS England. Exploring a conscious learning systems approach for integrated care systems. NHS England; 2021, unpublished.
- Healthcare Improvement Scotland. About quality management system [webpage]. Healthcare Improvement Scotland; no date (https://ihub.scot/improvement-programmes/quality-management-system/about-quality-management-system).
- NHS England and NHS Improvement. NHS Patient Safety Strategy. NHS England and NHS Improvement; 2019 (www.england.nhs.uk/wp-content/uploads/2020/08/190708_Patient_Safety_Strategy_for_website_v4.pdf).
- NHS Institute for Innovation and Improvement. Releasing Time to Care: The Productive Community Hospital: Patient Flow (MIU). NHS Institute for Innovation and Improvement; 2020 (www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2020/06/Productive-community-hospital-Patient-Flow.pdf).
- NEJM Catalyst. What is Patient Flow? NEJM Catalyst, 1 January 2018 (https://catalyst.nejm.org/doi/full/10.1056/CAT.18.0289).
- NHS Institute for Innovation and Improvement. Improvement Leaders’ Guide: Improving Flow: Process and Systems Thinking. NHS Institute for Innovation and Improvement; 2017 (www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/ILG-2.3-Improving-Flow.pdf).
- Health Foundation. Flow Coaching Academy [webpage]. Health Foundation; no date (www.health.org.uk/funding-and-partnerships/programmes/flow-coaching-academy).
- Health Foundation. Improving flow along care pathways: Learning from the Flow Coaching Academy programme. November 2020 (www.health.org.uk/publications/reports/improving-flow-along-care-pathways).
- Wise J. The BMJ Awards 2019: Innovation in Quality Improvement Team of the Year. BMJ. 2019; 365: I1653 (www.bmj.com/content/365/bmj.l1653).
- Flow Coaching Academy. The Flow Coaching Academy Roadmap for Improvement. Flow Coaching Academy; no date (www.flowcoaching.academy/UserFiles/File/New_Guidance_Documents/The_FCA_Roadmap_for_Improvement.pdf).
- BMJ. 237+ Million Medication Errors Made Every Year in England. BMJ; no date (www.bmj.com/company/newsroom/237-million-medication-errors-made-every-year-in-england).
- MHRA. Stage Three: Directive: Improving Medication Error Incident Reporting and Learning (Patient Safety Alert). NHS England; 2014 (www.england.nhs.uk/wp-content/uploads/2014/03/psa-sup-info-med-error.pdf).
- University of Nottingham. PRIMIS [webpage]. University of Nottingham; no date (www.nottingham.ac.uk/primis).
- Academic Health Science Network. PINCER [webpage]. Academic Health Science Network; no date (https://ahsn-nenc.org.uk/what-we-do/improving-population-health/medicines-optimisation/pincer).
- NHS England. House of Care – a framework for long term condition care [webpage]. NHS England; no date (www.england.nhs.uk/ourwork/clinical-policy/ltc/house-of-care).
- Department of Health. Long Term Conditions Compendium of Information (Third Edition). DoH; 2012 (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216528/dh_134486.pdf).
- Barnett NL. Medical adherence: Where are we now? A UK perspective. European Journal of Hospital Pharmacy. 2013; 21(3): 181–4 (www.researchgate.net/publication/273883281_Medication_adherence_Where_are_we_now_A_UK_perspective).
- National Institute for Health and Clinical Excellence. Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. NICE; 2009 (www.nice.org.uk/guidance/cg76/chapter/introduction#ftn.footnote_1).
- National Institute for Health and Care Research. Development and evaluation of an intervention to support adherence to treatment in adults with cystic fibrosis (ACtiF) [webpage]. NIHR; no date (www.journalslibrary.nihr.ac.uk/programmes/pgfar/RP-PG-1212-20015#).
- CFHealthHub. CFDigiCare [webpage] CFHealthHub; no date (www.cfhealthhub.com/cfdigicare).
- Wildman MJ, O’Cathain A, Maguire C, Arden MA, Hutchings M et al. Self-management intervention to reduce pulmonary exacerbations by supporting treatment adherence in adults with cystic fibrosis: A randomised controlled trial. Thorax. 2021; doi: 10.1136/thoraxjnl-2021-217594.
- NHS England. ‘Miracle’ Cystic Fibrosis Treatment for Children on the NHS. NHS England; 2022 (www.england.nhs.uk/2022/01/miracle-cystic-fibrosis-treatment-for-children-on-the-nhs).
- Bohmer R, Shand J, Allwood D, Wragg A, Mountford J. Learning Systems: Managing Uncertainty in the New Normal of Covid-19. NEJM Catalyst; 16 July 2020 (https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0318).
- UCL Partners. The bedside learning coordinator role captures insights and feedback to improve patient care, workplace efficiency and staff well-being [webpage]. UCL Partners; no date (https://uclpartners.com/work/the-bedside-learning-co-ordinator-role-capturing-and-acting-on-insights-from-the-front-line).
- Robson J, Boomla K, Hull S. Learning Health Systems. The exceptional potential of general practice. Watt G Ed. CRC Press; 2018.
- Wolfe I, Thompson M, Gill P, Tamburlini G, Blair M et al. Health services for children in Western Europe. The Lancet. 2013; 381(9873): 1224–34.
- Southwark Council. Children & Young People’s Health Partnership (Report for the Health and Well Being Board). Southwark Council; no date (https://moderngov.southwark.gov.uk/documents/s54753/Appendix%201%20Report%20for%20Health%20and%20Wellbeing%20Board.pdf).
- Newham JJ, Foreman J, Heys M, Cousens S, Lemer C et al. Children and Young People’s Health Partnership (CYPHP) Evelina London model of care: protocol for an opportunistic cluster randomised controlled trial (cRCT) to assess child health outcomes, healthcare quality and health service use. BMJ Open. 2019; 9: e027301.
- Children & Young People’s Health Partnership. About CYPHP [webpage]. CYPHP; no date (www.cyphp.org/about-cyphp).
- Children & Young People’s Health Partnership. Research & evaluation [webpage]. CYPHP; no date (www.cyphp.org/cyp-professionals/model-of-care/research-and-evaluation-moc).
- Crandall W, Kappelman MD, Colletti RB, Leibowitz I, Grunow JE et al. ImproveCareNow: The development of a pediatric inflammatory bowel disease improvement network. Inflammatory Bowel Diseases. 2011; 17(1): 450–7 (https://pubmed.ncbi.nlm.nih.gov/20602466).
- ImproveCareNow. Purpose & Success [webpage]. ImproveCareNow; 2022 (www.improvecarenow.org/purpose-success).
- PCORI. ImproveCareNow: a learning health system for children with Crohn’s disease and ulcerative colitis – phase I [webpage]. PCORI; no date (www.pcori.org/research-results/2013/improvecarenow-learning-health-system-children-crohns-disease-and-ulcerative-colitis-phase-i#project_summary).
- Pronovost P, Mathews S, Chute C, Rosen A. Creating a purpose driven learning and improving health system: The Johns Hopkins Medicine quality and safety experience. Learning Health Systems. 2016; 1(1): e10018.
- Department of Health and Social Care. Better, Broader, Safer: Using Health Data for Research and Analysis. DHSC; 2022 (www.gov.uk/government/publications/better-broader-safer-using-health-data-for-research-and-analysis).
- Maxwell E. Patient feedback: How effectively is it collected and used? Nursing Times. 2011; 116(12): 27–9 (www.nursingtimes.net/clinical-archive/patient-experience/patient-feedback-how-effectively-is-it-collected-and-used-16-11-2020).
- Wellings D, Thorstensen-Woll C. How does the health and care system hear from people and communities? The King’s Fund; 2022 (www.kingsfund.org.uk/publications/health-care-system-people-and-communities).
- Health Foundation. The Networked Data Lab [webpage]. Health Foundation; no date (www.health.org.uk/funding-and-partnerships/our-partnerships/the-networked-data-lab).
- Keith J, Grimm F, Steventon A. How Better Use of Data Can Help Address Key Challenges Facing the NHS. Health Foundation; 2022 (www.health.org.uk/publications/long-reads/how-better-use-of-data-can-help-address-key-challenges-facing-the-nhs).
- Bardsley M, Steventon A, Fothergill G. Untapped Potential: Investing in Health and Care Data Analytics. Health Foundation; 2019 (www.health.org.uk/publications/reports/untapped-potential-investing-in-health-and-care-data-analytics).
- Department of Health and Social Care. Data Saves Lives: Reshaping Health and Social Care with Data. DHSC; 2022 (www.gov.uk/government/publications/data-saves-lives-reshaping-health-and-social-care-with-data/data-saves-lives-reshaping-health-and-social-care-with-data).
- Comptroller and Auditor General. The Use of Digital Technology in the NHS. National Audit Office; 2020 (www.nao.org.uk/report/the-use-of-digital-technology-in-the-nhs).
- Department for Digital, Culture, Media and Sport. Data: A New Direction. DCMS; 2021 (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1022315/Data_Reform_Consultation_Document__Accessible_.pdf).
- Boyd KM. Ethnicity and the ethics of data linkage. BMC Public Health. 2007; 7: 318 (https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-7-318#:~:text=Linking%20health%20data%20with%20census,interests%20of%20ethnic%20minority%20groups).
- Taylor JA. The road to hell is paved with good intentions: The experience of applying for national data for linkage and suggestions for improvement. BMJ Open. 2020; 11(8): e047575.
- Smart A, Harrison E. The under-representation of minority ethnic groups in UK medical research. Ethnicity and Health. 2017; 22(1): 65–82.
- Ibrahim H, Liu X, Zariffa N, Morris AD, Denniston A. Health data poverty: an assailable barrier to equitable digital health care. The Lancet Digital Health. 2021; 3(4): E260–E265.
- Understanding Patient Data. Public Attitudes to Patient Data Use: A Summary of Existing Research. Understanding Patient Data; 2018 (https://understandingpatientdata.org.uk/sites/default/files/2018-08/Public%20attitudes%20key%20themes_0.pdf).
- Lai, A., et al. An Informatics Consult approach for generating clinical evidence for treatment decisions. BMC Medical Informatics and Decision Making. 2021; 21, [281].
- Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: Analyses of standalone and linked national databases. BMC Medicine. 2016; 14: 113 (https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0657-8).
- Asthma + Lung UK. Asthma deaths in Scotland highest for more than a decade. Asthma + Lung UK; no date (www.asthma.org.uk/about/media/news/asthma-deaths-in-scotland-highest-for-more-than-a-decade).
- Scottish Public Health Observatory. Asthma: key points [webpage]. Scottish Public Health Observatory; 2021 (www.scotpho.org.uk/health-wellbeing-and-disease/asthma/key-points).
- Asthma + Lung UK. Scotland’s leadership on using health data in research [webpage]. Asthma + Lung UK; 2016 (www.asthma.org.uk/support-us/campaigns/campaigns-blog/andrew-morris-scotland-blog).
- HDR UK. Learning public health system for preventing asthma attacks [webpage]. HDR UK; no date (www.hdruk.ac.uk/projects/learning-public-health-system-for-preventing-asthma-attacks).
- ORCHID. Incidence and prevalence of active asthma [webpage]. ORCHID; no date (https://orchid.phc.ox.ac.uk/index.php/asthma-dashboard).
- Tibble H, Tsanas A, Horne E, Horne R, Mizani M et al. Predicting asthma attacks in primary care: Protocol for developing a machine learning-based prediction model. BMJ Open. 2019; 9: e028375. (https://bmjopen.bmj.com/content/bmjopen/9/7/e028375.full.pdf).
- Nwaru BI, Friedman C, Halamka J, Sheikh A. Can learning health systems help organisations deliver personalised care? BMC Medicine. 2017; 15: 177.
- Zhang J, Tüshaus L, Martínez NN, Moreo N, Verastegui H et al. Data integrity-based methodology and checklist for identifying implementation risks of physiological sensing in mobile health projects: Quantitative and qualitative analysis. JMIR mHealth and uHealth. 2018; 6(12): e11896 (https://mhealth.jmir.org/2018/12/e11896).
- Hardie T, Mahadeva S, Horton T. Realising the Benefits of Technology in Health Care. Health Foundation; 2021 (www.hdruk.ac.uk/wp-content/uploads/2021/05/Realising-the-benefits-of-technology-in-healthcare.pdf).
- House of Commons Science and Technology Committee. Robotics and Artificial Intelligence: Fifth Report of Session 2016–17. House of Commons; 2016 (https://publications.parliament.uk/pa/cm201617/cmselect/cmsctech/145/145.pdf).
- The King’s Fund. Activity in the NHS [webpage]. The King’s Fund; 2022 (www.kingsfund.org.uk/projects/nhs-in-a-nutshell/NHS-activity).
- Khanbhai M, Anyadi P, Symons J, Flott K, Darzi A et al. Applying natural language processing and machine learning techniques to patient experience feedback: A systematic review. BMJ Health and Care Informatics. 2021; 28(1): e100262.
- NHS England. Population health and the Population Health Management Programme [webpage]. NHS England; no date (www.england.nhs.uk/integratedcare/what-is-integrated-care/phm).
- Encompass. About encompass [webpage]. Health and Social Care Northern Ireland; 2022. https://encompassni.hscni.net/about-encompass/?csrt=12489003636674274673
- Sutton RT, Pincock D, Baumgart DC, Sadowksi DC, Fedorak RN et al. An overview of clinical decision support systems: Benefits, risks, and strategies for success. npj Digital Medicine. 2020: 17 (www.nature.com/articles/s41746-020-0221-y).
- Digital Health. Building Clinical Decision Support into the Heart of the EPR. Digital Health; 2022 (www.digitalhealth.net/2022/03/building-clinical-decision-support-into-the-heart-of-the-epr).
- Gallier S, Topham A, Nightingale P, Garrick M, Woolhouse I et al. Electronic prescribing systems as tools to improve patient care: A learning health systems approach to increase guideline concordant prescribing for venous thromboembolism prevention. BMC Medical Informatics and Decision Making. 2022; 22: 121 (https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-022-01865-y).
- Coleman J, Hodson J, Brooks H, Rosser D. Missed medication doses in hospitalised patients: A descriptive account of quality improvement measures and time series analysis. International Journal for Quality in Healthcare. 2013; 25(5): 564–72 (www.ncbi.nlm.nih.gov/pmc/articles/PMC3786625).
- Coleman J, Hodson J, Ferner RE. Deriving dose limits for warnings in electronic prescribing systems: Statistical analysis of prescription data at University Hospital Birmingham, UK. Drug Safety. 2012; 35(4): 291–8 (https://pubmed.ncbi.nlm.nih.gov/22263779).
- Kassakian SZ, Yackel TR, Deloughery T, Dorr DA. Clinical decision support reduces overuse of red blood cell transfusions: Interrupted time series analysis. The American Journal of Medicine. 2016; 129(6): e13–e20 (https://pubmed.ncbi.nlm.nih.gov/26873112).
- Carding N. Electronic decision support ‘to be the norm for all clinicians’ under NHSE plan. 2022. HSJ (www.hsj.co.uk/technology-and-innovation/electronic-decision-support-to-be-the-norm-for-all-clinicians-under-nhse-plan/7031829.article)
- Varghese J, Kleine M, Gessner S, Sandmann, S, Dugas M. Effects of computerized decision support system implementations on patient outcomes in inpatient care: a systematic review. Journal of the American Medical Informatics Association. 2018; 25(5): 593-602 (www.ncbi.nlm.nih.gov/pmc/articles/PMC7646949).
- Wyatt J. Scott P. Computable knowledge is the enemy of disease. BMJ Health and Care Informatics, 2020; 27:e.100200 (https://informatics.bmj.com/content/bmjhci/27/2/e100200.full.pdf).
- Shaikh F, Dehmeshki J, Bisdas S, Roettger-Dupont D, Kubassova O et al. Artificial intelligence-based clinical decision support systems using advanced medical imaging and radiomics. Current Problems in Diagnostic Radiology. 2021; 50(2): 262–7 (https://pubmed.ncbi.nlm.nih.gov/32591104).
- Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: Students’ experiences and learning outcomes. Medical Education Online. 2021; 26(1) (www.tandfonline.com/doi/full/10.1080/10872981.2021.1953953).
- House of Commons Health and Social Care Committee. Clearing the Backlog Caused By the Pandemic. House of Commons; 2021 (https://committees.parliament.uk/publications/8352/documents/85020/default).
- Department of Health and Social Care. Health Secretary Sets Out Ambitious Tech Agenda. DHSC; 2022 (www.gov.uk/government/news/health-secretary-sets-out-ambitious-tech-agenda).
- NHS England. Digital Maturity Assessment 2017. NHS England; 2019 (https://data.england.nhs.uk/dataset/digital-maturity-2017/resource/fc6354cc-1f0b-4b47-afcc-ecdb9ce8b988).
- Health Foundation. Health and Care Bill [webpage]. Health Foundation; 2021 (www.health.org.uk/what-we-do/health-and-care-bill).
- Honeyman M, Dunn P, McKenna H. A Digital NHS? An Introduction to the Digital Agenda and Plans for Implementation. The King’s Fund; 2016 (www.kingsfund.org.uk/sites/default/files/field/field_publication_file/A_digital_NHS_Kings_Fund_Sep_2016.pdf).
- NHS Confederation and NHS Providers. Digital Transformation in Community Health Services. NHS Confederation and NHS Providers; 2021 (www.nhsconfed.org/sites/default/files/2021-11/Digital-transformation-in-community-health-services.pdf).
- Digital Social Care and Skills for Care. Digital Maturity in the Social Care Sector – Quantitative Research. Digital Social Care and Skills for Care; 2021 (www.digitalsocialcare.co.uk/new-report-on-the-digital-maturity-of-the-social-care-sector-published).
- Mahadeva S, Hardie T, Horton T. A complex patchwork of programmes. Health Foundation; 2021 (www.health.org.uk/news-and-comment/blogs/a-complex-patchwork-of-programmes).
- The AHSN Network. 2019. HealthTech Landscape Overview. The AHSN Network; 2019 (www.ahsnnetwork.com/wp-content/uploads/2019/02/02585-HealthTech-Report-LOWER-RES.pdf).
- Accenture. Half of Digital Health Start-ups will Fail within Two Years of Launch, Accenture Finds. Accenture; 2015 (https://newsroom.accenture.com/news/half-of-healthcare-it-start-ups-will-fail-within-two-years-of-launch-accenture-finds.htm).
- Guo C, Ashrafian H, Ghafur S, Fontana G, Gardner C et al. Challenges for the evaluation of digital health solutions—a call for innovative evidence generation approaches. npj Digital Medicine. 2020; 3: 110.
- Asthana S, Jones R, Sheaff R. Why does the NHS struggle to adopt eHealth innovations? A review of macro, meso and micro factors. BMC Health Services Research. 2019; 19: 984 (https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-019-4790-x).
- Murray E, Hekler EB, Andersson G, Collins LM, Doherty A et al. C. Evaluating digital health interventions: Key questions and approaches. American Journal of Preventive Medicine. 2017; 51(5): 843–51 (www.ncbi.nlm.nih.gov/pmc/articles/PMC5324832).
- Guo C, Ashrafian H, Ghafur S, Fontana G, Gardner C et al. Challenges for the evaluation of digital health solutions – A call for innovative evidence generation approaches. Digital Medicine. 2020; 3(110): 1-14.
- Campbell B, Campbell M, Dobson L, Higgins J, Dillon B et al. 2018. Assessing the value of innovative medical devices and diagnostics: The importance of clear and relevant claims of benefit. International Journal of Technology Assessment in Health Care. 2018; 34(4): 419–24 (www.cambridge.org/core/journals/international-journal-of-technology-assessment-in-health-care/article/assessing-the-value-of-innovative-medical-devices-and-diagnostics-the-importance-of-clear-and-relevant-claims-of-benefit/93D039B8F67C78312DF1CEBB642CFF19).
- McLellan A. Technology ‘tsunami’ creates funding challenge. HSJ. 2022 (www.hsj.co.uk/policy-and-regulation/technology-tsunami-creates-funding-challenge-says-regulator-chief/7031737.article).
- ABHI. Making it happen: delivering future innovation in healthtech. ABHI; 2021.
- Hardie T, Horton T, Warburton W. Switched On. Health Foundation; 2021 (www.health.org.uk/publications/reports/switched-on).
- Mahadeva S. Putting health tech into practice. Seven key factors for successful implementation. Health Foundation 2021. (www.health.org.uk/news-and-comment/blogs/putting-health-tech-into-practice).
- Lloyds Bank. Essential Digital Skills Report 2021. Lloyds Bank; 2021 (www.lloydsbank.com/assets/media/pdfs/banking_with_us/whats-happening/211109-lloyds-essential-digital-skills-report-2021.pdf).
- Healthwatch. Locked Out: Digitally Excluded People’s Experiences of Remote GP Appointments. Healthwatch; 2021 (www.healthwatch.co.uk/sites/healthwatch.co.uk/files/Digital%20Exclusion%20v4.pdf).
- Horton T, Hardie T, Mahadeva S, Warburton W. Securing a positive health care technology legacy from COVID-19. Health Foundation; 2021 (www.health.org.uk/publications/long-reads/securing-a-positive-health-care-technology-legacy-from-covid-19).
- Diabetes UK. Diabetes statistics [webpage]. Diabetes UK; no date (www.diabetes.org.uk/professionals/position-statements-reports/statistics).
- National Institute for Health and Care Excellence. NICEimpact: Diabetes. NICE; 2018 (www.nice.org.uk/media/default/about/what-we-do/into-practice/measuring-uptake/impact-diabetes.pdf).
- NHS England. NHS Prevention Programme cuts chances of Type 2 diabetes for thousands. NHS England; 2022 (www.england.nhs.uk/2022/03/nhs-prevention-programme-cuts-chances-of-type-2-diabetes-for-thousands/#:~:text=Evidence%20has%20shown%20that%20the,effective%20in%20the%20long%2Dterm).
- Imperial College Health Partners. RADAR Better Care Catalyst Project – view from the patient [webpage]. Imperial College Health Partners; 2021 (https://imperialcollegehealthpartners.com/radar-better-care-catalyst-project-view-from-the-patient).
- Discover-NOW. Predicting Complications in Diabetes Patients. Discover-NOW; no date (https://discover-now.co.uk/case-study/predicting-complications-in-diabetes-patients).
- Collen D. RADAR Better Care Catalyst Project – view from the patient and practitioner. HDR UK; 2021 (www.hdruk.org/news/radar-better-care-catalyst-project-patient-blog).
- Cystic Fibrosis Trust. Cystic fibrosis FAQs [webpage]. Cystic Fibrosis Trust; no date (www.cysticfibrosis.org.uk/what-is-cystic-fibrosis/faqs).
- Mayo Clinic. Cystic fibrosis [webpage]. Mayo Clinic; no date (www.mayoclinic.org/diseases-conditions/cystic-fibrosis/symptoms-causes/syc-20353700).
- Cystic-Fibrosis.com. Managing and Treating Cystic Fibrosis Exacerbations. Cystic-Fibrosis.com; no date (https://cystic-fibrosis.com/treatment/exacerbation).
- Smyth A, Elborn, JS. Exacerbations in cystic fibrosis: Management. Thorax. 2006; 63(2): 180–4.
- Magic Bullet. Project Breathe [webpage]. Magic Bullet; no date (https://magicbullet.co.uk/#projectbreathe).
- HDR UK. Health Data Research UK Better Care programme completes three ‘Catalyst Projects’. HDR UK; 2021 (www.hdruk.ac.uk/news/health-data-research-uk-better-care-programme-completes-three-catalyst-projects).
- Bach D. How a Cloud-Based Solution is Transforming Care for People with Cystic Fibrosis. Microsoft; 2020 (https://news.microsoft.com/innovation-stories/project-breathe-cystic-fibrosis).
- Cystic Fibrosis Trust. Project Breathe: Accelerating and Adapting to Manage COVID-19. Cystic Fibrosis Trust; 2020 (www.cysticfibrosis.org.uk/news/project-breathe-accelerating-and-adapting-to-manage-covid19).
- Li L, Grimshaw, J, Nielsen C, Judd M, Coyte P et al. Evolution of Wenger's concept of community of practice. Implementation Science. 2009; 4(11):1-8 (https://link.springer.com/content/pdf/10.1186/1748-5908-4-11.pdf).
- Jones B, Kwong E, Warburton W. Quality Improvement Made Simple. Health Foundation; 2021 (www.health.org.uk/publications/quality-improvement-made-simple).
- Royal College of Physicians. Final Evaluation Report: Scaling Up Improvement Round 2: Scaling Up for Safety: Standardising the Lessons Learnt from HipQIP. Royal College of Physicians; 2019.
- Kaleidoscope Health and Care. Case Study: The National Children & Young People’s Diabetes Network. Kaleidoscope Health and Care; 2020 (https://s30454.pcdn.co/wp-content/uploads/Case-Study-The-National-Children-Young-Peoples-Diabetes-Network.pdf).
- National Voices. Voice for Improvement [webpage]. National Voices; 2022 (www.nationalvoices.org.uk/our-work/Voicesforimprovement).
- Health Foundation. Common ambition [webpage]. Health Foundation; no date (www.health.org.uk/funding-and-partnerships/programmes/common-ambition).
- Gabbay J, le May A, Connell C, Klein JH. Skilled for Improvement? Health Foundation; 2014 (www.health.org.uk/sites/default/files/SkilledForImprovement_fullreport.pdf).
- Voice Global. About us [webpage]. Voice Global; no date (www.voice-global.org/about).
- Bentley C, Browman G, Poole, B. Conceptual and practical challenges for implementing the communities of practice model on a national scale – a Canadian cancer control initiative. BMC Health Services Research. 2010; 10: 3 (www.ncbi.nlm.nih.gov/pmc/articles/PMC2820037).
- BMA. An NHS under pressure [webpage]. BMA; 2022 (www.bma.org.uk/advice-and-support/nhs-delivery-and-workforce/pressures/an-nhs-under-pressure).
- NHS Confederation. Fall in staff morale a real cause for concern. NHS Confederation; 2022 (www.nhsconfed.org/news/fall-staff-morale-real-cause-concern).
- Ocloo J, Garfield S, Franklin BD, Dawson S. Exploring the theory, barriers and enablers for patient and public involvement across health, social care and patient safety: A systematic review of reviews. Health Research Policy and Systems. 2021; 19: 8 (https://health-policy-systems.biomedcentral.com/articles/10.1186/s12961-020-00644-3).
- Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. The Patient – Patient-Centered Outcomes Research. 2014; 7: 387–95 (https://link.springer.com/article/10.1007/s40271-014-0065-0).
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: Implications from a qualitative analysis of the scientific literature. Learning Health Systems. 2021; 6(2): e10287 (https://onlinelibrary.wiley.com/doi/full/10.1002/lrh2.10287).
- Psek W, Davis FD, Gerrity G, Stametz R, Bailey-Davis L et al. Leadership perspectives on operationalizing the learning health care system in an integrated delivery system. The Journal for Electronic Health Data and Methods. 2016; 4(3): 1233 (www.ncbi.nlm.nih.gov/pmc/articles/PMC5019321).
- Burgess N, Currie G, Crump B, Dawson A. Leading change across a healthcare system: how to build improvement capability and foster a culture of continuous improvement, Report of the Evaluation of the NHS-VMI partnership. Warwick Business School; 2022 (publication forthcoming).
- Dorgan S, Layton D, Bloom N, Homkes R, Sadun R et al. Management in Healthcare: Why Good Practice Really Matters. McKinsey & Company; no date (https://cep.lse.ac.uk/textonly/_new/research/productivity/management/PDF/Management_in_Healthcare_Report.pdf).
- Horwitz LI, Kuznetsova M, Jones SA. Creating a learning health system through rapid-cycle, randomized testing. New England Journal of Medicine. 2019; 381(12): 1175–9.
- Braithwaite J, Herkes J, Ludlow K, Testa L, Camprell G. Association between organisational and workplace cultures, and patient outcomes: Systematic review. BMJ Open. 2017; 7: e017708.
- O’Sullivan OP, Chang NH, Baker P, Shah A. Quality improvement at East London NHS Foundation Trust: The pathway to embedding lasting change. International Journal of Health Governance. 2021; 26(1): 65–72.
- Jones B, Horton T. The Improvement Journey. Health Foundation; 2019 (www.health.org.uk/publications/reports/the-improvement-journey).
- NHS England. The NHS Long Term Plan. NHS England; 2019 (www.longtermplan.nhs.uk).
- Academy of Medical Royal Colleges. Quality Improvement: Training for Better Outcomes. Academy of Medical Royal Colleges; 2016.
- Davey P, Thakore S, Tully V. How to embed quality improvement into medical training. BMJ. 2022; 376: e055084.
- Mannion R, Davies H. Understanding organisational culture for healthcare quality improvement. BMJ. 2018: 363.
- Bohmer R, Shand J, Allwood D, Wragg A, Mountford J. Learning systems: Managing uncertainty in the new normal of Covid-19. NEJM Catalyst; 16 July 2020 (https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0318#).
- Dixon-Woods M, Baker R, Charles K, Dawson J, Jerzembek G et al. Culture and behaviour in the English National Health Service: Overview of lessons from a large multimethod study. BMJ Quality & Safety. 2014; 23(2): 106–15.
- Vaughn VM, Saint S, Krein SL, Forman JH, Meddings J, Ameling J et al. Characteristics of healthcare organisations struggling to improve quality: Results from a systematic review of qualitative studies. BMJ Quality & Safety. 2019; 28: 74–84.
- Gabbay J, le May A, Wright D. Able to improve? The skills and knowledge NHS front-line staff use to deliver quality improvement: findings from six case studies. Health Foundation; 2020 (www.health.org.uk/research-projects/able-to-improve-how-front-line-staff-transform-and-use-quality-improvement-skills).
- Royal College of Physicians. National Hip Fracture Database annual report. Royal College of Physicians; 2018 (www.rcplondon.ac.uk/projects/outputs/nhfd-annual-report-2018#:~:text=As%20a%20result%2C%20hip%20fracture,of%20the%20whole%20NHS%20budget).
- Health Foundation. Saving lives through safer hip fracture care. The Health Foundation; no date (www.health.org.uk/funding-and-partnerships/programmes/saving-lives-through-safer-hip-fracture-care#:~:text=A%20fifth%20of%20people%20who,practice%2C%20evidence%2Dbased%20care.)
- Royal College of Physicians. HipQIP: scaling up project [webpage]. Royal College of Physicians; no date (www.rcplondon.ac.uk/projects/outputs/hipqip-scaling-project).
- Health Foundation. Scaling Up for Safety: Standardising the Lessons Learnt from a Hip Fracture Quality Improvement Programme. Health Foundation; no date (www.health.org.uk/improvement-projects/scaling-up-for-safety-standardising-the-lessons-learnt-from-a-hip-fracture).
- Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough. Institute for Healthcare Improvement; 2003 (www.ihi.org/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreakthroughImprovement.aspx).
- Bliss. Prematurity statistics in the UK [webpage]. Bliss; no date (www.bliss.org.uk/research-campaigns/neonatal-care-statistics/prematurity-statistics-in-the-uk).
- Cerebral Palsy Guidance. Premature birth and cerebral palsy [webpage]. Cerebral Palsy Guidance; 2022 (www.cerebralpalsyguidance.com/cerebral-palsy/risk-factors/premature-birth).
- National Institute for Health and Care Research. New film showcases research to improve life chances of pre-term babies. NIHR; 2020 (https://arc-w.nihr.ac.uk/news/new-film-showcases-research-to-improve-life-chances-of-pre-term-babies).
- National Institute for Health and Care Research. Finding the best way to scale up a perinatal quality improvement initiative: the PReCePT Study. NIHR; no date (https://arc-w.nihr.ac.uk/research/projects/preventing-cerebral-palsy-in-pre-term-babies-the-precept-study).
- Health Foundation. Written evidence submitted by the Health Foundation (MSE0066). Health Foundation; 2020 (https://committees.parliament.uk/writtenevidence/11132/pdf).
- The AHSN Network. PReCePT: reducing cerebral palsy through improving uptake of magnesium sulphate in preterm deliveries [webpage]. The AHSN Network; 2022 (www.ahsnnetwork.com/case-study/precept-reducing-cerebral-palsy-through-improving-uptake-of-magnesium-sulphate-in-preterm-deliveries).
- The AHSN Network. PReCePT resources [webpage]. The AHSN Network; no date (www.ahsnnetwork.com/about-academic-health-science-networks/national-programmes-priorities/precept/precept-resources).
- Health Foundation. PReCePT2: learning from Scaling Up Improvement project. Health Foundation; 2020 (www.youtube.com/watch?v=QhJtKnZz8BY&ab_channel=TheHealthFoundation).
- National Institute for Health and Care Research. PReCePT study informs new Quality Improvement innovation toolkit. NIHR; 2021 (https://arc-w.nihr.ac.uk/news/precept-study-informs-new-quality-improvement-innovation-toolkit).
- Health Foundation. PReCePT2: reducing brain injury through improving uptake of magnesium sulphate in preterm deliveries [webpage]. Health Foundation; no date (www.health.org.uk/improvement-projects/precept2-reducing-brain-injury-through-improving-uptake-of-magnesium-sulphate).
- The AHSN Network. PReCePT: preventing cerebral palsy in preterm babies [webpage]. The AHSN Network; no date (www.ahsnnetwork.com/about-academic-health-science-networks/national-programmes-priorities/precept).
- NHS Providers. Understanding interoperability [webpage]. NHS Providers; no date (https://nhsproviders.org/making-the-right-technology-decisions/understanding-interoperability).
- Learning Health Community. The Learning Healthcare System Toolkit. Learning Health Community; no date (https://lhstoolkit.learninghealthcareproject.co.uk).