Acknowledgements
The authors would like to thank a number of people who have helped with this research and the production of this report. These include a range of experts in scaling and spread whom we had the benefit of consulting: David Albury, Suzie Bailey, Amanda Begley, Dan Berelowitz, Helen Bevan, Anna Burhouse, Alison Cracknell, Helen Crisp, David Fillingham, Aidan Fowler, Michael Hallsworth, Abigail Harrison, Axel Heitmueller, Anne Kilgallen, Tom Ling, Annie Laverty, Alison Lovatt, Michael McCooe, Ross Millar, Greg Nathan, Rozenn Perrigot, Julie Reed, Mike Richards, Brian Robson, Beverley Slater, Charles Tallack, Anna Watson, Mary Wells, Tom Woodcock, and particularly Mary Dixon-Woods, whose research over many years has done so much to develop our understanding of this field.
We would also like to extend special thanks to all of the Health Foundation grant holders and project teams we worked with during the course of this research, including Steve Ariss, Tania Barnes, Nick Blake, James Breakwell, Bridget Callaghan, Mercy Darty, Jessica Deighton, Paul Dodd, Tom Downes, Tim Draycott, Alastair Ferraro, James Findlay, Bev Fitzsimons, Hugh Gallagher, Colin Gelder, Andy Henwood, Michael Holland, Jocelyn Hopkins, Peter Lachman, Sonia Lee, Claire Morrissey, Michael Nation, Clare Peckham, Lesley Peers, Mike Reed, Alan Taylor, Helen Ward and Martin Wilkie.
Finally, we wish to thank our colleagues Jennifer Dixon, Tim Gardner, Sarah Henderson, Bryan Jones, Penny Pereira and Tracy Webb for their helpful comments and insights in producing this report. Particular thanks to Oli Smithson, Insight & Analysis Support Officer at the Health Foundation, for his assistance in conducting the survey and preparing the manuscript.
The views expressed in this report are those of the authors alone.
Key terms used in this report
Intervention: An intended change to existing practices or services that aims to improve health care. An intervention may consist of a single component or several different components that each contribute towards the intervention’s aims.
Innovator: The individual, team or organisation that developed the idea for the intervention or that first implemented it within the UK.
Spread programme: An initiative aiming to achieve the replication of the intervention in new sites or settings.
Programme leader: The individual, team or organisation leading the spread of the intervention to new sites or settings. (Where the innovator is leading the spread of an intervention they have developed, they will also be the programme leader.)
Adopter: An individual, team or organisation other than the innovator that implements the intervention in a different site or setting to the one in which it was originally developed.
Adaptation: A change made by an adopter to the intervention, compared to the innovator’s original version, as they implement the intervention in a new site or setting.
Codification: A description of the intervention, along with any supporting materials, aimed at enabling others to reproduce it. Codifying an intervention requires thinking through what adopters will need to know in order to reproduce it successfully, for example, what is core to making the intervention work and what can be adapted.
Innovation and improvement: New approaches, practices, treatments, technologies and services that aim to improve health care. The analysis in this report applies to both innovation and improvement; on some occasions we use both terms together, and on others we use one as a shorthand for both. (As described above, we use the term ‘innovator’ to refer to the individual, team or organisation that developed the idea for the intervention, whether it is an ‘innovation’ or an ‘improvement’.) Improvement, including formal quality improvement (QI) using a structured method, is often used to describe incremental change within an existing service model, whereas innovation can be used to mean disruptive change that creates a new service model. Furthermore, innovation is often viewed as a discrete, one-off change, whereas improvement is often viewed as iterative and ongoing. Nevertheless, both innovation and improvement tend to involve one or more interventions (see above) and it is such interventions that are the main focus of our analysis here.
Scaling and spread: Activity that results in an intervention being replicated across multiple sites. Scaling, which is a subset of spread, refers to an initiative to replicate an intervention specifically through a higher-level organisation or geographical entity (such as a professional body or government agency); but spread can also happen through horizontal connections between adopters, without the involvement of a higher-level entity. The analysis in this report applies to both scaling and spread; we sometimes use both terms together, though more commonly ‘spread’ is used as a shorthand for both.
Executive summary
What this report is about and why it matters
How to spread new ideas and effective practices from one organisation to another to improve care and reduce unwarranted variations in performance is one of the central challenges facing the NHS.
For decades, centuries even, people have debated the problem of the slow spread of innovations in health care. A classic example is the incorporation of citrus fruit into sailors’ diets to prevent scurvy – demonstrated by James Lancaster in 1601 and again by James Lind in 1747, but not adopted by the British navy until 1795. And since Everett Rogers published his classic work The Diffusion of Innovations in 1962, much debate and scholarship on innovation has focused on the factors that affect whether people take up innovations or not and how quickly they do so.
This report focuses on a different problem, one that has received far less attention, but which we believe is equally pressing: that when an individual, team or organisation does take up a new innovation it may not work as well as it did first time round – something we see particularly with complex health care interventions that seek to make improvements in clinical processes or pathways. We therefore set out to investigate not the factors affecting the uptake of innovations in health care, but the factors affecting their successful uptake. We do this in several ways, reviewing the literature on this problem, drawing out lessons from Health Foundation projects and evaluations, and also interviewing key actors – innovators and adopters, who provide vital insights from the front line of health care, as well as expert stakeholders involved in supporting scaling and spread.
Indeed, this ‘replicability problem’ – the challenge of replicating the impact of a new intervention as well as its external form – is arguably the more urgent question for the public sector, and for the NHS in particular, where all manner of mechanisms exist to encourage uptake, and indeed to mandate it in the last resort. But the last 70 years of NHS history have shown that mandating action does not automatically bring about the desired change in outcomes.
Achieving that is much harder. It requires teams on the ground to adapt and implement a new intervention in ways that will enable it to work in their own setting. Staff may need to develop new skills or learn to use new techniques. There may be a need for culture change, relationship building, new ways of working or undoing entrenched habits – none of which can be achieved purely through compulsion.
Framed in this way, it is clear that while the invention of new technologies, practices and models of care are exciting moments in health care, invention itself is only half the story. People sometimes fall into the trap of thinking that when an idea has been successfully demonstrated or piloted then the hard work is done. But exploiting the full potential of a new idea requires successful replication at scale – and this takes time, skill, resources and imagination.
So as policymakers and system leaders draw up the anticipated long-term plan for the NHS in England, it is important that debate is not restricted simply to identifying areas for improvement and potential solutions. The challenge is to get the solutions working well everywhere.
This report discusses some of the changes in thinking and approach needed to tackle this challenge, drawing on lessons from the Health Foundation’s programmes to support the spread of innovation and improvement in health care.
One part of the answer is changing the way we think about what is actually being spread. In the case of a discrete health care intervention, does our conceptualisation of the intervention encompass the full range of factors necessary for its success, such as underlying behaviours or cultural factors, the skills and capabilities required, the methodology for implementation, and so on? This applies not just to process innovations, quality improvement approaches or new service models, but also to interventions using new health technologies or medical devices – where there is often a tendency to focus on the technology itself, even though its effectiveness will depend on the skills, behaviours and organisational cultures of those using it.
Another part of the answer involves designing programmes that will better support the spread of new interventions. Do these programmes generate consensus on the new idea and buy-in from those adopting it? Do they provide for sufficient support during implementation to enable the idea to be successfully reproduced? Factors such as these could have major implications for the success of programmes to scale up new ideas or reduce variation in the NHS.
We hope the analysis and insights in the report will be relevant both for innovators and adopters, and also for those designing and leading spread programmes. The latter includes those overseeing local programmes, whether they are run by commissioners, academic health science networks (AHSNs), regional and national improvement bodies or professional networks. It also includes policymakers and system leaders overseeing national change programmes, such as Getting It Right First Time or RightCare, and national bodies whose remit includes spreading innovation and improvement, such as the National Institute for Health and Care Excellence, royal colleges and professional bodies.
The central argument of the report
Our central argument is that successfully spreading complex health care interventions will require packaging them up in more sophisticated ways and designing programmes to spread them in more sophisticated ways.
The point of departure for the report’s analysis is to note that the success of a complex intervention is likely to depend heavily on its context: the underlying systems, culture and circumstances of the environment in which it is implemented. This means that adopting a complex intervention and making it work in a new setting is not a straightforward matter; in fact, as many of the Health Foundation’s grant holders tell us, it can be extremely hard work. Successful implementation may require adaptation of the intervention or a long journey to build new relationships, shift the prevailing team culture or develop new skills.
Recognising the influence of context highlights the important role adopters play in translating interventions to new settings. This poses a challenge for traditional approaches to spreading innovation, which tend to assume that once an innovator has developed an idea and successfully piloted it, it can then be ‘diffused’ and taken up by others in a straightforward way. By contrast, we argue that reproducing a complex intervention at scale is a much more distributed effort, often involving a good deal of creativity and reinvention from those taking it up, with the intervention itself sometimes undergoing substantial revision and refinement in the process.
We argue that designing spread programmes that can meet the challenges of adoption will therefore place a greater focus on adopters, to some extent reversing the conventional focus on the innovator. This requires ‘codifying’ interventions in ways that support adopters to adapt them appropriately. It requires designing programmes in ways that build adopters’ commitment to implementing the intervention. It requires mechanisms such as peer networks to capture and share the learning that adopters generate as they tackle implementation challenges. Above all, a greater focus on adopters requires building their capability and readiness for implementation and providing them with the resources, time and space needed to do the hard work of translating the original idea to their own setting.
Chapter summary
Chapter 1 gives a brief overview of the ‘replicability problem’.
Key points:
- When initially successful interventions are spread to new settings, they may fail to achieve the same impact, or indeed any impact at all.
- One explanation for this may be that interventions are not being conceptualised and described in ways that enable them to be successfully reproduced in new contexts, and programmes to spread interventions are not being organised in ways that adequately support adopters to reproduce them.
- These challenges become especially acute with complex health care interventions, such as innovations and improvements in clinical processes and pathways.
Chapter 2 looks at why the complexity of many health care interventions poses challenges for spreading them.
Key points:
- Complex interventions tend to have certain properties that make codifying and replicating them difficult: they are social, context-sensitive and dynamic in nature.
- Various approaches and schools of thought exist for analysing complexity, such as realist evaluation or complex adaptive systems theory, but they all represent routes for capturing and responding to these properties of complex interventions.
- Insufficient appreciation of complexity can lead to mistakes and misconceptions in attempts to codify and spread interventions. These can include failing to consider the social as well as technical components of the intervention, failing to distinguish between the instrumental and expressive effects of intervention components, or failing to recognise capability-building as an integral part of the intervention.
Chapter 3 considers some approaches to codifying complex health care interventions in ways that can support effective replication.
Key points:
- Some approaches to codification aim for ‘tight’ descriptions of the intervention through comprehensive and detailed accounts, for example, specifying the methods for implementation or the social mechanisms and behaviours required for success. Other approaches aim for ‘loose’ descriptions, focusing less on the details of each intervention component and more on the ability of adopters to formulate their own versions of these components in their own setting, for example, by setting out the underlying principles and goals, the intervention’s theory of change, or the skills and capabilities required. Furthermore, tightening and loosening approaches can sometimes be blended.
- Whichever approach is taken will have implications for the concept of fidelity to the intervention, as well as for the broader approach to spreading the intervention.
- Innovators and programme leaders should be aware of different possible approaches to conceptualising and describing interventions; they should be seen as a standard part of the innovator’s toolkit.
Chapter 4 looks at the initial spread process and at how early adopters generate new learning about an intervention as they implement it in new contexts.
Key points:
- As innovators cannot necessarily ‘see’ their own context, introducing a new intervention into a diverse range of sites can generate fresh insights into what is (and isn’t) significant for making it work. This allows the innovator to revise the description of the intervention accordingly, and in some cases to refine the intervention itself.
- There may be value in recognising this testing and revision phase as a formal part of the innovation cycle in health care, distinct from attempts to spread interventions at later stages of maturity.
- This has implications for the design of the initial spread process, in terms of setting expectations, selecting initial adopter sites to ensure diversity, fostering good relationships between the innovator and the initial adopters, and ensuring that mechanisms are in place to capture and share new learning.
Chapter 5 looks at some consequences for the design of large-scale spread programmes.
Key points:
- Spread programmes need to be designed in ways that build and maintain adopters’ commitment to implementation, including seeking consensus on both the problem and the proposed solution.
- These phenomena have important psychological, behavioural and social dimensions that should be considered when designing spread programmes. Important areas where behavioural insights can inform the design of programmes include peer leadership, peer communities and adopter ownership.
- Successful spread also relies on adopters’ ability to implement the intervention and on them having sufficient opportunity to do so. This implies that spread programmes need to build adopter readiness and capability (for example, through providing training or enabling relationship building), include appropriate support for implementation (for example, funding to cover the upfront costs of adoption, or assistance with analytics and evaluation), and be based on realistic timescales.
The conclusion draws out the implications for policymakers and those overseeing spread programmes.
Key points:
- Adopters make a crucial contribution to the successful spread of new ideas, both through the hard work involved in adoption and their role in generating new learning about an intervention as it spreads.
- This perspective challenges conventional notions of the division of labour between innovator and adopter. It also challenges the ‘knowledge pipeline’ model of innovation, which sees new knowledge as generated purely by the innovator and which casts adopters as passive recipients of this knowledge during the diffusion process.
- There needs to be greater emphasis on the role and status of adopters within spread programmes, both in terms of how interventions are codified and how programmes are designed. Beyond specific spread programmes, policymakers can ensure health care providers are better equipped for adoption more generally by supporting them to build their improvement capability.
The replicability problem
Although replicating successful health care interventions in new contexts is clearly essential for maximising the benefits of new ideas and innovations for patients, it is also a well-recognised challenge. A commonly seen phenomenon is that when initially successful interventions are spread to new settings they may fail to achieve the same impact, or indeed any impact at all.
The phenomenon of surgical safety checklists provides an example of these variable fortunes. While the introduction of such checklists around the world has sometimes been associated with significant reductions in surgical complications and mortality, in other cases it has not led to improvements, even when compliance with the checklist has been high.
On other occasions, even when some impact is achieved, there can be a reduction in effectiveness or ‘voltage drop’ as initiatives are replicated. In the field of social programmes more broadly, this phenomenon was nicknamed the ‘Iron Law’ by evaluator Peter Rossi in the 1980s, who argued that as a new initiative is implemented across more and more settings, the impact will tend toward zero.
There may sometimes be straightforward explanations for this ‘replicability problem’, such as a failure by adopters to adhere to the intervention protocols. In recent years, however, there has been growing interest in a deeper set of explanations: that we may not be describing interventions in ways that enable them to be successfully reproduced in new contexts, and that we may not be designing programmes to spread interventions in ways that adequately support adopters to reproduce them.
This problem of replicability is different from the one that has traditionally preoccupied innovation research, namely, identifying what drives the uptake of new ideas. Much of this wider research stems from Everett Rogers’ seminal work on the diffusion of innovations, first published in 1962, which explored the properties of innovations and social systems affecting uptake. A more recent and highly influential contribution is the review of the literature on the diffusion of innovations in service organisations by Trish Greenhalgh and colleagues, who highlight the roles played by a large range of factors in driving the adoption of innovation in health services. Here, by contrast, we are concerned not simply with uptake but with the challenge of successful or effective uptake and the factors that affect whether someone can replicate an intervention’s impact when they do take it up.
Perhaps it is not surprising that this proves such a challenge. For an adopter to be able to reproduce an intervention successfully, they need to understand how they can translate the idea into their own setting, they need to know just what matters for the intervention to work in this new setting, and they need to have the opportunity, motivation and capability to implement it. None of these things are trivial, yet it is surprising how often they are taken for granted in initiatives to scale up and spread new ideas.
This goes beyond the well-known phenomenon of incomplete descriptions of interventions, with crucial details often omitted from reports, hampering effective replication. For example, Hoffman and colleagues analysed reports from a large sample of randomised trials of non-drug interventions and found that more than half (61%) were not described in sufficient detail to enable replication of the intervention in practice. Rather, the issue may be whether we are conceptualising the interventions themselves in the right way. For example, is the conceptualisation broad enough to incorporate all those aspects of context that might impinge upon the intervention’s effectiveness? Or does it strike the right balance between the ‘what’ and the ‘how’?
And these challenges become especially acute with complex health care interventions, particularly innovations in clinical processes and pathways of the kind that the Health Foundation has supported over many years, such as improvements in patient flow or hospital discharge. This includes the use of new technologies such as apps and medical devices; these are sometimes viewed as ‘simple’ interventions, but their effectiveness will depend on the skills, behaviours and cultures of those using them, and the evolution of new ways of working to maximise their benefits.
This report presents lessons and insights on how to tackle these challenges that have emerged from Health Foundation programmes and research. It begins by considering why complexity poses problems for conceptualising and replicating interventions, before moving on to consider the implications both for how we describe interventions and how we support their spread.
The Health Foundation’s programmes to support scaling and spread
Over the last decade, the Health Foundation has run five major programmes to support the spread of innovations and improvements in health care. These have included programmes explicitly designed to support teams to spread complex interventions to new sites (such as the Scaling Up programme), as well as programmes seeking to improve health care at scale more generally (such as the Closing the Gap series of programmes) but which nevertheless included projects of the kind we are concerned with here (namely, those engaged in spreading defined interventions to specific sites).
Table 1 gives the details of these programmes dating from 2009 to 2017. Since then, a third round of the Scaling Up initiative has been launched, supporting a further seven teams.
Table 1: Details of Health Foundation programmes on scaling and spread 2009–2017
|
Programme |
When it ran |
Aim |
Investment (including evaluation) |
Number of projects supported |
|
Closing the Gap through Clinical Communities |
2009–2012 |
To support the uptake of improvement interventions through established clinical networks |
£5.3m |
11 |
|
Closing the Gap through Changing Relationships |
2010–2013 |
To support organisations to implement interventions that improve the relationship between people and health services |
£3.9m |
7 |
|
Closing the Gap in Patient Safety |
2014–2016 |
To scale evidence-based patient safety interventions through groups of collaborating organisations |
£4.0m |
9 |
|
Spreading Improvement |
2014–2017 |
To support teams to spread their improvement interventions through creating contexts and infrastructures that support diffusion |
£2.2m |
5 |
|
Scaling Up (Rounds 1 & 2) |
2014–2017 |
To support teams to implement tested improvement interventions at scale, in partnership with organisations that can support regional or national spread |
£7.4m |
13 |
With one team receiving a grant in more than one of these programmes, there are a total of 44 discrete projects across the five programmes, listed in Appendix 1.
How we have learned from the Health Foundation’s programmes
The insights presented in this report emerged from interviews with many Health Foundation grant holders and partners during 2017 and 2018.
We also benefited from close engagement with several of the projects supported by the Health Foundation’s programmes, through interviews with innovators, adopters and evaluators conducted in 2017 and 2018. Three of these projects are presented as detailed case studies in this report:
- Shared Haemodialysis Care, a project to spread an approach to haemodialysis that gives patients the opportunity to take a greater role in their own care, beginning in 12 dialysis units and subsequently spreading to a further seven units in England between 2016 and 2018, as part of the Health Foundation’s Scaling Up programme (see Chapter 3).
- Respiratory Innovation: Promoting Positive Life Experience (RIPPLE), a project to spread a new model of community clinic for tackling social isolation and improving health for people with chronic obstructive pulmonary disease (COPD) to six sites across the East and West Midlands between 2015 and 2018, as part of the Health Foundation’s Spreading Improvement programme (see Chapter 4).
- Situational Awareness For Everyone (SAFE), a project to implement patient safety huddles – initially in 12 paediatric units and subsequently in a further 16 units in England between 2014 and 2017 – as part of the Health Foundation’s Closing the Gap in Patient Safety programme (see Chapter 5).
In addition, we conducted a survey of innovators and adopters from the Health Foundation’s spread-related programmes during December 2017 and January 2018, the results of which are presented at various points throughout this report. Further information on the survey is given in Box 1.
Finally, throughout the report, we draw on examples and evaluations from the Health Foundation’s improvement work more broadly, both from the spread-related programmes listed above and other relevant programmes such as Safer Clinical Systems (2008–2016) and Flow Cost Quality (2010–2012).
Box 1: Our survey of innovators and adopters from the Health Foundation’s programmes
Over the last decade, the Health Foundation has funded five major spread-related programmes, which together have supported 44 different projects. After analysing each of these projects, we concluded that 26 could be categorised as aiming to spread a defined intervention or approach to specific adopter sites – the paradigm of scaling and spread with which this report is concerned. The other 18 projects took different approaches to achieving improvements at scale, such as by influencing national guidelines or through improvement collaboratives supporting different interventions in different sites.
To investigate the views of programme participants, we conducted surveys of the innovators and, separately, the adopters from these 26 projects, throughout December 2017 and January 2018, using Qualtrics software. We received responses from 21 innovators and 42 adopters.
The survey asked them about their experiences of trying to spread or implement an intervention as part of the relevant Health Foundation programme; sought their reflections on the process with hindsight; and invited their views on general questions about spread and adoption within the context of their experiences in the Health Foundation programme. The results are illustrated at various points throughout this report.
The complexity of modern health care interventions
The previous chapter highlighted how reproducing the desired impact of a new health care intervention can be a particular challenge in the case of complex interventions. This chapter explores why complexity poses problems for spreading and replicating interventions.
There is no single definition of a complex health care intervention, though analyses of complexity tend to highlight certain features:
- the presence of multiple components, either independent or interacting
- context-embeddedness: complex interventions are built on and interact with the underlying systems, culture and circumstances of the environment in which they are implemented. Indeed, some interventions will be so embedded in their context that it can be hard to distinguish the intervention from its implementation in a specific context.
- intricacy of causal pathways (that is, the ways in which interventions achieve their effects): these pathways can be multiple and interacting, possibly containing feedback loops, with the effects of some intervention components reinforcing or moderating the effects of others.
Of course, these characteristics are not unrelated. A greater number of intervention components and potential interactions between them will tend to increase the number of ways in which the intervention might supervene upon and interact with the underlying organisational context. And all of this has the potential to increase the complexity of the causal pathways by which the intervention achieves its effects.
The indefinite and potentially contested nature of these characteristics does not always lend itself to drawing hard and fast distinctions about which interventions are complex and which are not. Rather, it might make more sense to think about degrees of complexity. For example, we can imagine a spectrum ranging from simple to highly complex. At one end might be an innovation like a pill, or an intervention that replaces one type of pill with a more effective one. At the other end could be an intervention like a rehabilitation programme, which has multiple components delivered in a range of settings and relies upon a significant degree of patient co-production and behaviour change. In between, one can imagine a range of intervention types that share these characteristics to varying degrees.
Figure 1: Intervention complexity spectrum
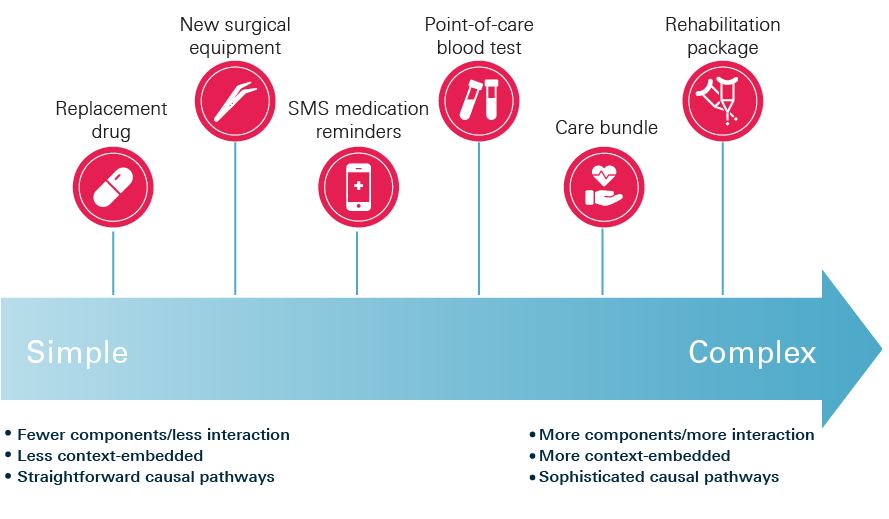
Note: Figure 1 illustrates some common types of health care interventions and where they might typically sit on this spectrum. However, we do not intend to suggest that all instances of a particular type of intervention will be of equal complexity.
A failure to consider the full range of issues involved in implementing an intervention could lead one to underestimate its degree of complexity, as illustrated in the case of the Sepsis Six care bundle, described in Box 2. And the more we underestimate the complexity of an intervention, the more likely we are to underestimate the challenges involved in replicating it in new contexts.
Specifically, complex interventions tend to have certain properties (related to the characteristics outlined above) that explain why codifying and replicating them can be so difficult.
First, complex interventions are social, in that they are co-produced and delivered by health care staff, patients and carers. This implicates the attitudes, behaviours, relationships and organisational cultures of those adopting an intervention in its success or failure. Understanding how particular intervention components work may therefore require understanding the social mechanisms that facilitate them, and successful replication may require adopters to re-create these social dynamics in their own setting.
Second, the fact that complex interventions are context-embedded means they are context-sensitive: an intervention’s success will be influenced by aspects of the organisational and wider context in which it is implemented, including the social and relational elements described above. This means it may be necessary for the intervention description to set out which aspects of context influence its effectiveness, and how, in order to aid replication. And to the extent that aspects of organisational context may differ from one location to the next, successful replication may require adaptation of the intervention. As a result, the same intervention may look different in different contexts; for example, when interventions are co-designed with patients, local patient priorities will shape the intervention (and, in the process, challenge standardisation). This, in turn, means that the intervention description will need to capture which components are ‘core’ to making the intervention work and their tolerance to alteration across different settings – what needs to remain invariant versus what can be changed and to what extent.
Third, complex interventions are dynamic, in that the ‘systems’ (people, teams, organisations, etc.) that implement them can learn and self-organise, and the contexts in which they are implemented can throw up new issues requiring a response from those involved., This means that a complex intervention may evolve over time and in unpredictable ways. What is more, its fate may rely heavily on the adopter’s ability to navigate these dynamics and adapt. Indeed, the degree of adaptation and responsiveness required will have consequences for the extent to which the success of the intervention is seen to reside in the specific intervention components themselves versus the agency and capability of the adopter.
Box 2: The complexity of apparently simple interventions: the Scottish national collaborative programme on sepsis
The varying success of an intervention across different contexts can sometimes be a symptom that its underlying complexity has gone unrecognised. One example is the ‘Sepsis Six’ clinical care bundle, which focuses on six key tasks required to treat a patient with sepsis within one hour of diagnosis, including prompt administration of antibiotics and oxygen. Studies have shown significant variation in the effectiveness with which sepsis care bundles are implemented. In light of this, an ethnographic study sought to understand the realities of implementing Sepsis Six on the front line through an investigation of the Scottish national collaborative programme on sepsis, which ran from 2012 to 2014.
Superficial consideration might suggest the Sepsis Six bundle comprises six steps. However, the researchers identified some 48 steps typically required for implementation of the six key tasks. Some required significant input from several staff, making completion within one hour challenging. For example, to effectively administer high flow oxygen, the staff member had to find a doctor and get oxygen prescribed, assess the patient for COPD, gather equipment, wash their hands, explain the reason for the procedure and gain patient consent, administer oxygen, check for comfort and document the procedure.
Across the six key tasks, there were interdependencies inherent in the sequencing of different steps and a lack of synchronisation could arise when staff had to resolve competing demands or manage interruptions. Some steps had social as well as technical dimensions, relying on collaboration between multiple groups of professionals; for instance, nurses in most cases were reliant on doctors to prescribe antibiotics, and on other nurses to check and sign, before they could administer the antibiotics.
The researchers concluded that the challenges of prompt and reliable implementation of the Sepsis Six bundle involve ‘the socio-technical complexity of completing interdependent tasks requiring multiple individuals in different professional groups in a frenetic environment characterised by competing priorities’, and that improving performance ‘requires attention to problems of coordinating tasks, workflow, accountability and expertise’. So, while packaging together different care processes into ‘bundles’ may well be helpful for ensuring consistency, it is clear that effective implementation requires grasping the complexity of the underlying processes and creating working environments that enable them to be delivered.
Analysing complexity
A range of theoretical and practical approaches have evolved for analysing complexity in both health care and health promotion.
One example is realist evaluation, which attempts to understand how an intervention’s outcomes were produced in terms of the underlying mechanisms that drive behaviour and the influence of context on how actors respond. Specifically, realist analysis considers which configurations of context, mechanism and outcome offer the most plausible explanation for the intervention’s effects (here, ‘mechanisms’ are not intervention components but underlying processes triggered by the context that causes or facilitates the intervention’s outcomes).
Realist evaluation is one of a broader set of theory-based approaches that seek to explain how interventions work by elucidating the intervention’s ‘programme theory’ or ‘theory of change’. All interventions are seen as underpinned by a theory – whether the practitioner is aware of it or not – but without making this explicit, it is difficult to fully understand the mechanisms that underpin the intervention’s effectiveness. By uncovering how an intervention works, theory-based approaches can lead to revisions in the innovator’s understanding of their intervention, sometimes in unexpected ways. Box 3 provides an example from the Health Foundation programme Closing the Gap in Patient Safety.
Another type of approach comes from complexity science and complex adaptive systems theory., These disciplines consider an intervention in terms of a system of interdependent agents whose interaction gives rise to emergent, system-wide patterns of behaviour that cannot be reduced solely to the impact of its component parts. Furthermore, such complex systems can self-organise, giving rise to unpredictability, and meaning interventions can evolve.
Different schools of thought handle issues of complexity in contrasting ways. Some view complexity primarily as a property of the intervention and seek to draw relevant background conditions and enabling factors into the conceptualisation of the intervention itself, whereas others tend towards seeing complexity as a feature of the context in which the intervention is embedded. Another point of contrast concerns how and at what level to capture causality: for some, the way to navigate the context dependency of complex interventions is to describe the function of different components within the intervention’s theory of change rather than their specific form; others question whether it is possible to capture causality in this way, because of the difficulty of linking individual components to outcomes, and seek instead to understand the system-level changes triggered by the intervention.,
Whether or not the divisions between the different schools of thought that exist are helpful is debatable. Nevertheless, while we do not seek to play down the philosophical differences between them, in our view they all represent routes for capturing the same underlying features of complex interventions highlighted here: an intervention’s social dimension, how it embeds in its context and how it may evolve over time as those implementing it learn and adapt.
Box 3: Understanding how interventions work: surviving sepsis in Northumbria
Scrutiny of the factors underpinning an intervention’s success can reveal that interventions sometimes have their effects in unexpected ways, requiring those codifying or implementing the intervention to revise their theory of change.
In 2013, Northumbria Healthcare NHS Foundation Trust had a higher than expected mortality rate for people admitted with sepsis, which led to sepsis being identified as a priority for improvement. Supported through the Health Foundation’s programme Closing the Gap in Patient Safety, in 2014 the trust began a quality improvement project to reliably screen patients for sepsis and, where sepsis was identified, to treat them using the Sepsis Six care bundle.
Within 15 months, the project resulted in an increase in patients receiving Sepsis Six within the first hour from fewer than 10% to approximately 60%. Analysis of results from 8,000 screened patients suggest that an estimated 158 lives were saved from reductions in mortality rates.
However, through the project evaluation, the team was surprised to discover that raising staff awareness of the possibility of sepsis through reliable screening ultimately made the greatest contribution to reducing mortality rates; patients assessed using the screening tool were shown to have a 21% lower mortality rate than those for whom the tool had not been used. The subsequent use of Sepsis Six following screening contributed to a further reduction in mortality rates, but not of the same magnitude.
The team also learned about the mechanisms by which the screening tool had this effect. As well as providing a clear pathway for the steps to be taken when sepsis was identified, the tool also promoted team coherence, not least by legitimising nurses’ role in escalating patients and initiating treatment. This was a significant change in practice, as previously nursing staff had simply reported deterioration and awaited instructions from the doctors.
Some common mistakes and misconceptions
The features of complexity considered here pose challenges not just for understanding how an intervention achieves its effects but also for trying to spread it to other settings. The challenges involved can be illustrated by considering some common mistakes and misconceptions that can occur in attempts to codify and replicate interventions.
The first is the belief that the technical components form the ‘hard’ core of an intervention, while the social components are ‘soft’ – that is, discretionary or more open to variation. In fact, the social components may be essential for the intervention to work. This was illustrated in a study of a successful programme to reduce central venous catheter bloodstream infections in intensive care units (ICUs) across Michigan. Some contemporary accounts had viewed the intervention as a simple checklist of five technical components, such as using chlorhexidine for skin preparation and barrier precautions during catheter insertion. However, the study found that the checklist also had an important social function in promoting adherence to these technical practices, because the programme did not simply ask ICUs to use the checklist, but also specified that every catheter insertion should be monitored by a nurse, who would immediately raise concerns if the protocol was not followed. Importantly, this requirement presupposed a restructuring of professional relationships, flattening the traditional hierarchy within the ICU, and it would only work if nurses were able and willing to intervene. In turn, the study found that unwavering support from senior consultants was crucial in enabling nurses to act in this way. Whether one regards such social and relational dynamics as aspects of ‘context’ or of the intervention itself, exposing and understanding them can be essential for effective replication. And this is true not just for interventions that may outwardly appear ‘social’, such as the creation of new teams or ways of working, but also for the use of new technologies or devices; in such cases, the benefits rarely come purely from the technology itself, but rather from the technology being successfully embedded in the human environment of a care process or pathway.
A second possible misconception is to confuse the direct or instrumental effects of intervention components with their expressive or symbolic effects. For example, the study of the Michigan programme cited above found that the requirement for ICUs to create a dedicated trolley containing all the items required for successful catheter insertion not only had instrumental benefits in averting delays but also expressive ones: it signalled the organisation’s commitment to infection control and heightened awareness of the programme. Another example, from the Health Foundation’s Safer Clinical Systems programme, comes from a project which aimed to reduce medication errors on a hospital ward. One component activity, nicknamed the ‘dabber audit’, involved dabbing medication charts with different coloured ink stampers to indicate whether they were correct or needed further action. Originally intended to simplify the data collection process, its visibility meant it came to be regarded as a powerful motivator of behaviour and an educational tool – and as its function mutated, it became possible to see the dabber audit as an intervention in its own right. Sensitivity to the expressive as well as the instrumental functions of an intervention component in a particular context can be important for describing it in a way that supports others to replicate the same effects.
A third possible misconception is a failure to recognise capability-building as an integral part of the intervention. Again, the perception of checklists as a catch-all provides an illustration. While the introduction of surgical safety checklists around the world has sometimes been associated with reductions in surgical complications and mortality, when their use was mandated in Ottawa in 2010, an evaluation found no such improvements, even though compliance with the checklist was high. The researchers noted that, unlike other instances where positive effects of using surgical safety checklists had been observed, the introduction of the checklist in Ottawa had not included team training on how to use it. They suggest a greater effect might have occurred had training been provided.
***
These social, context-sensitive and dynamic properties of complex interventions mean that substantial effort and creativity may be required by adopters to translate a new intervention into their own setting and make it work successfully. The next three chapters explore three specific challenges this creates for spread programmes:
- how to codify complex interventions in a way that can support their implementation in new contexts (Chapter 3)
- how to incorporate learning from attempts to implement the intervention in new contexts in order to revise and refine it (Chapter 4)
- how to design spread programmes in ways that build adopters’ commitment and support their work in translating the intervention into their own context (Chapter 5).
Codifying complex health care interventions
The previous chapter looked at how complexity poses challenges for codifying health care interventions in ways that can support effective replication. This chapter considers some possible approaches to codification in response.
Adequate description of an intervention’s technical components is, of course, often critical for effective replication. Research studies highlight the role that poor quality descriptions of technical components can play in preventing successful spread. This has led to the development of approaches to assist innovators and evaluators in producing more robust intervention descriptions – for example, the TIDieR framework.
However, the analysis in the previous chapter suggests that successfully replicating complex interventions also requires codifying them in ways that go beyond a description of the technical components and allow adopters to navigate the underlying social, contextual and dynamic forces.
Various approaches to codification are evident in the evaluation and implementation science literature, as well as in practice in the Health Foundation’s programmes. These are characterised by two contrasting impulses we would describe as ‘tightening’ and ‘loosening’.
Some approaches seek to ‘tighten’ the intervention description in response to the challenges of codification by attempting more comprehensive or fine-grained specifications than simply a straightforward description of the intervention’s technical components. This could include specifying the method for implementing particular components, for example, ‘lean’ principles. Another possible tightening approach is to set out relevant social mechanisms and dynamics in addition to technical components; the case of PROMPT, described in Box 4, could be viewed as an example of this kind of approach.
Other approaches, by contrast, seek to ‘loosen’ the description of the intervention by focusing less on specifying the details of each component and more on the ability of adopters to formulate their own versions of these components in their own setting. This includes approaches that focus on the theory of change underpinning the intervention and that see fidelity as replicating the function that components play within this theory of change rather than their original form. It also includes approaches that focus on the underlying principles and goals of the intervention and allow adopters to work towards their own way of fulfilling these – an example of which can be seen in the case study of Shared Haemodialysis Care at the end of this chapter. Another possible loosening approach is to focus on building the knowledge, skills and capabilities that adopters require to re-create the intervention’s effects in their own setting. In the language of intervention manuals, tightening approaches aim to lengthen the manual, while loosening approaches effectively support the adopter to write their own version.
Box 4: PROMPT: the importance of considering the social aspects of an intervention
PROMPT (Practical Obstetric Multi-Professional Training) is a one-day, multi-professional training course developed by a team at Southmead Hospital in Bristol. It uses simulation models to address the clinical and behavioural skills required by teams responding to obstetric emergencies. It has been associated with significant improvements in care outcomes, including a 50% reduction in babies born starved of oxygen and a 70% reduction in babies suffering from shoulder dystocia. The training course includes a detailed manual setting out technical components, such as emergency drills for dealing with obstetric haemorrhage, along with some principles for running the training, such as multi-professional participation and the use of props and patient actors.
While PROMPT has spread widely, consistent replication of the successes seen at Southmead has been harder. For example, implementation of PROMPT in one Australian state showed more modest improvements, and not all sites were able to implement it fully. This suggests that the training and the accompanying technical proficiency may not by themselves fully explain the outcomes seen at Southmead. Indeed, a survey of organisations adopting PROMPT suggests that how well a unit implements the package is related to their underlying safety culture and attitudes. This implies that PROMPT is not simply a technical intervention but a ‘social’ one, too, and that successful implementation relies on factors such as the values, behaviours and relationships within the organisations implementing it.
The Health Foundation is now funding new research to characterise the mechanisms underlying the improvements seen at Southmead. The research will also develop and test an additional ‘implementation package’ that incorporates an intervention to support the norms, behaviours and systems that need to be in place to reproduce Southmead’s safety outcomes.
Tightening and loosening approaches both represent attempts to absorb the context-dependent and social nature of complex interventions in order to support their successful implementation. Both also seek to reconcile the need for creativity and constraint, but via different routes. ‘Tight’ descriptions attempt to draw social and contextual factors into the intervention protocols, though in doing so tend to highlight the capabilities required for successful implementation. ‘Loose’ descriptions, by contrast, focus on helping adopters adapt the intervention to fit their own context, though in doing so make them ‘own’ the constraints within which they need to operate.
Whichever approach is taken will have implications for the concept of fidelity. Tight descriptions multiply the number of factors guiding faithful replication of the intervention, and can increase the likelihood that the ultimate form of the replicated intervention will resemble its original incarnation. Employing looser descriptions, by contrast, may well mean that fidelity is seen to reside in features of the intervention other than its original form, for example, in faithfulness to the goals of the intervention or the underlying principles, or in the use of a particular methodology to re-create it. As discussed earlier, loosening strategies also include theory-based approaches to codification, which see fidelity as residing in the function particular components play within the intervention’s theory of change. An example of a theory-based approach to fidelity, from the evaluation of the Health Foundation’s Safer Clinical Systems programme, is described in Box 5.
Box 5: Fidelity and adaptation in the Safer Clinical Systems programme
Safer Clinical Systems was a programme run by the Health Foundation between 2008–2016 to improve the safety and reliability of health care. The programme approach aimed to improve patient safety not by imposing pre-defined solutions on organisations, but by developing their capacity to diagnose system-level weaknesses and introduce interventions to address them. The tools and techniques used were mostly imported from other industries, then adapted and customised for health care. A prominent feature of the programme was its goal of changing the way organisations approached safety, from the prevailing reactive, incident-based approach to a more proactive, risk-based one.
The evaluation of Safer Clinical Systems identified the programme’s theory of change as consisting of two broad components: a stepwise method made up of diagnostic, implementation, measurement and reporting phases; and a proactive and collegial approach to safety. In the final phase of the programme, the evaluators assessed fidelity in terms of divergence from this theory of change. They outlined two types of divergence, which they termed ‘principled deviations’ and ‘conspicuous departures’. Principled deviations ‘served to address local needs and contextual pressures, but did not affect the two core elements of the theory of change’. Conspicuous departures, however, altered the core elements at the heart of the approach and transformed it into something different.
Principled deviations tended to be ‘functional’, allowing teams to overcome local constraints and maintain momentum in their work. For instance, during the diagnostic phase, one team renamed some of the tools and revised the language within them in response to concerns that they were off-putting for staff, while nevertheless maintaining the proactive, collegial and stepwise nature of the approach. The evaluators also saw these principled deviations as attempts by teams to gain ownership of the work and embed it into organisational practice.
In another site, however, the diagnostic phase was conducted by external consultants rather than permanent team members – a conspicuous departure from the programme approach, which had emphasised local ownership. The evaluation concluded that ‘the relationship between fidelity to and effectiveness of the Safer Clinical Systems was not straightforward, but there were indications that principled deviations led to satisfactory outcomes, while more conspicuous departures did not’.
Innovator views on tightening and loosening
In our survey of innovators from Health Foundation programmes, nine out of ten (91%) said adopters had made adaptations to the intervention during implementation, and nearly half of these innovators (47%) said they had seen instances of changes being made that had given them concerns about fidelity. So, we were interested to see their views on the best route for ensuring effective implementation.
Figure 2: Survey of innovators – adaptations made by adopters

As described above, a ‘tightening’ approach aims to specify more granular and detailed information about the intervention in order to reduce the risk of important factors being missed or changes being made that breach fidelity. A ‘loosening’ approach, by contrast, focuses less on the specific details of intervention components and more on the underlying goals, and on the capability of adopters to re-create their own version of the intervention in their own setting.
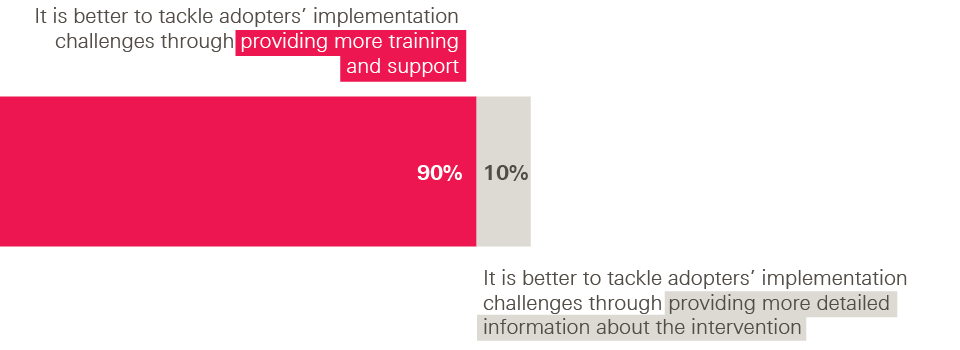
What did our innovators think of these approaches? Overwhelmingly, they favoured a loosening approach. When asked to choose between two statements, 90% of innovators chose ‘It is better to tackle adopters’ implementation challenges through providing more training and support to implement the intervention’, with only 10% choosing ‘It is better to tackle adopters’ implementation challenges through providing more detailed information about the intervention’.
Figure 3: Survey of innovators – tackling implementation challenges
This split was mirrored in innovators’ responses to a separate question, discussed in Chapter 5, about what, with hindsight, would have made the biggest difference for supporting adoption. The most popular options were capability-oriented – doing more in advance to support adopter readiness and providing more training and support – while the option of providing a more detailed written description of the intervention was one of the least popular.
***
There are merits in all of these approaches to intervention description and a priority for improvement research is to investigate which might work best in which circumstances. Interviews with grant holders engaged in the Health Foundation’s programmes highlight both advantages and disadvantages of tightening and loosening strategies. Tight descriptions that seek greater prescription may highlight important factors that support effective replication, but in doing so may make it harder for adopters to feel a strong sense of agency in shaping the intervention. Such approaches could also be open to the accusation that they are seeking a level of determinism over the link between components and outcomes that might be hard to attain in practice. Conversely, loose descriptions that seek greater abstraction potentially allow an adopter more scope for creative responses to contextual issues, but may also make adoption more challenging and risky. Indeed, providing too little concrete information can be daunting for potential adopters, who may yearn for clearer guidance at the outset.
Perhaps for these reasons, successful cases of codification often employ blends of these techniques. For example, specifying the function of different components within a theory of change could be combined with identifying the social and relational factors required to enable those components to function successfully, and which therefore have to be reproduced. Or a capability-building approach could be combined with detailed prescription of intervention components or implementation methods. An example of the latter, illustrated in Box 6, can be seen in the Flow Coaching Academy, which supports teams to improve flow along condition-based pathways using a training approach. A blended model can also be seen in franchising approaches to spreading innovation, (see Box 7), in which detailed manualisation is combined with ongoing training and support. So tightening and loosening strategies are not necessarily mutually exclusive, and can be simultaneously applied to different aspects of interventions.
Box 6: The Flow Coaching Academy
The Flow Coaching Academy supports teams to improve patient flow along condition-based pathways (such as stroke or acute paediatrics), using team coaching and ‘flow’ principles. It was developed from the learning gained in two previous Health Foundation-funded programmes: Flow Cost Quality and the Sheffield Microsystems Coaching Academy.41
Rather than prescribing specific intervention components or outcomes, the Academy instead trains individuals to use a ‘roadmap’ to coach flow improvement within their own organisations. An expert faculty delivers the one-year action learning curriculum at monthly sessions, which involves training in technical skills (such as process mapping) and relational skills (such as resolving difficult situations). This capability-building is combined with the ‘Big Room’ methodology (based on the ‘Oobeya process’, originally developed by Toyota) – a regular, standardised meeting that brings together staff and patients involved in a care pathway to iteratively test ideas, monitor data and progress, discuss issues, share experiences and agree next steps.
Work is now underway to develop a network of 10–12 such academies across the UK.
Box 7: Social franchising
Social franchising enables an adopter organisation to deliver a proven intervention or idea to agreed standards under a franchise agreement. The intervention is packaged up for franchisees to replicate, usually in the form of a manual, accompanied by training and support; in return, the franchisee pays a fee to cover the costs of the franchise operation and shares data and other information with the franchisor. The intervention manual usually sets out the essential components of the intervention, while permitting appropriate local flexibility where this is required for successful implementation.
As a method of replication, franchising sits in the middle of the spectrum in terms of the degree of affiliation between innovator and adopter. At one end of the spectrum, spread could be achieved through the growth of a single organisation, where ownership remains with the innovator; at the other end are approaches with no relationship whatsoever between innovator and adopter, for example where ideas are disseminated through publication and independently picked up by others. Franchising approaches sit somewhere in between: they require a degree of affiliation between franchisor and franchisee through the franchise agreement – both for the accountability of the franchisee to the franchisor and also for the support provided by the franchisor to the franchisee. As such, franchising has the potential to offer greater levels of support to adopters than some approaches to spread, and also greater control to the innovator to ensure fidelity to the original model. Different franchise operations strike different balances between these elements.
It is this combination of manualisation with training and implementation support that makes franchising a good example of a blended approach to codification and replication. It can permit tight specification of interventions and help ensure fidelity, while also recognising the importance of building adopter capability and providing ongoing support.
During 2018 and 2019, the Health Foundation will be testing the feasibility of using social franchising and licensing methods to spread complex health care interventions. Through the programme Exploring Social Franchising and Licensing, four teams are being funded to scale their intervention using these techniques. These range from a pharmacist-led IT intervention (PINCER) for reducing medication errors in general practice prescribing, led by the University of Nottingham, to an integrated care model (the Pathway model) to ensure homeless patients admitted to hospital have access to the care and support they need, led by the homeless health care charity Pathway. There will be a significant evaluation component to the programme, to generate wider learning on the applicability of franchising techniques to health care interventions.
In most discussions of innovation, the innovation itself – the thing that is to be spread – is taken as a given. The implication of the discussion here, by contrast, is that the notion of a health care intervention is to some extent a more moveable feast; it can be conceptualised in a variety of ways (as a set of component activities, capabilities, methods, principles, or a combination of these) and at varying levels of generality or specificity. As the examples above illustrate, exactly how an innovator chooses to characterise their intervention will have implications not only for the notion of fidelity, but also for the appropriate method for spreading the intervention. For example, a capability-based approach may lend itself to using training as a route to spread, whereas a theory-of-change approach will require a process that supports adopters to design their own corresponding version of the intervention.
Adopter views on flexibility versus prescription
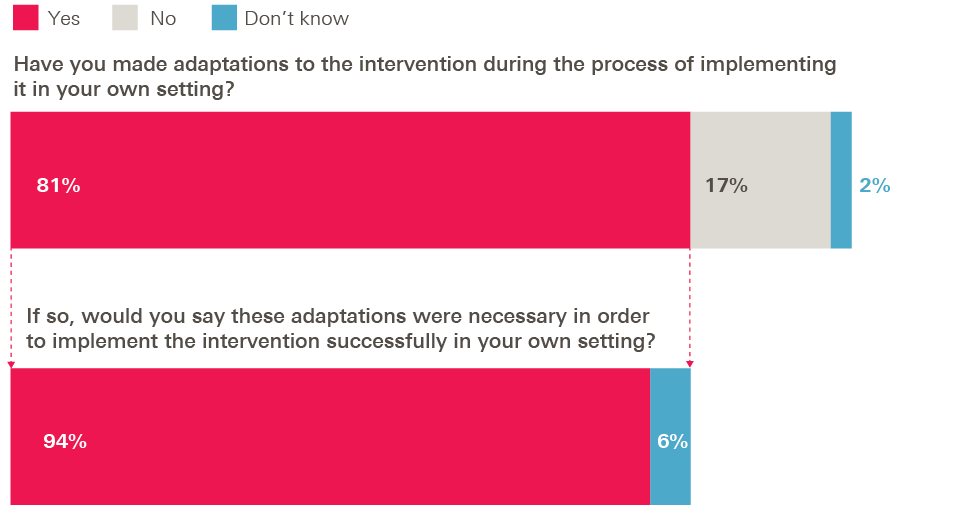
Our survey found mixed views among adopters of the right balance between prescription and flexibility. On the one hand, 81% of adopters in Health Foundation programmes said they had made adaptations to the intervention they were adopting, and nearly all of these adopters (94%) said the adaptations had been necessary in order to implement the intervention successfully in their own setting.
Figure 4: Survey of adopters – adaptations during implementation
And in response to a further question (discussed in more detail in Chapter 5) about what would have made the biggest difference in helping them adopt the intervention, adopters tended to favour capability-building over greater specification: more opportunities to share learning, more support to ensure adopter readiness and more training were the most popular options, while providing a more detailed written description of the intervention was the least popular.
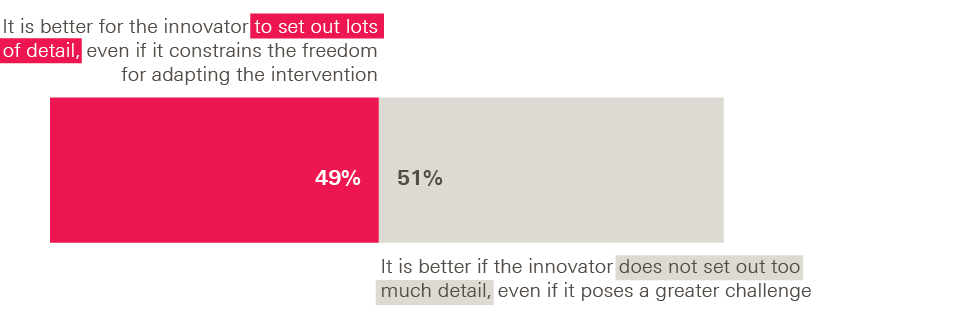
On the other hand, adopters emphasised in interviews that there is a balance to be struck; it can be daunting to implement a new intervention with insufficient information and guidance from the innovator. This sentiment also emerged in our survey, where adopters were evenly split on whether specifying lots of detail was a good thing or not. When asked to choose, 49% favoured the statement ‘It is better if the innovator/programme leader sets out lots of detail about the intervention in order to help others implement it, even if this constrains the freedom for adapting it to new settings’. The other 51% chose ‘It is better if the innovator/programme leader does not set out too much detail about the intervention in order to allow others sufficient freedom to adapt it, even if this poses a greater challenge for those implementing it’.
Figure 5: Survey of adopters – optimum level of detail
In a related question, three-quarters of adopters (76%) favoured the statement ‘The innovator is generally the ideal person to lead the initial spread process because they know most about the intervention’, with only a quarter (24%) choosing ‘The innovator is not generally the ideal person to lead the initial spread process because they are too wedded to their original design’.
Figure 6: Survey of adopters – leadership of the initial spread process
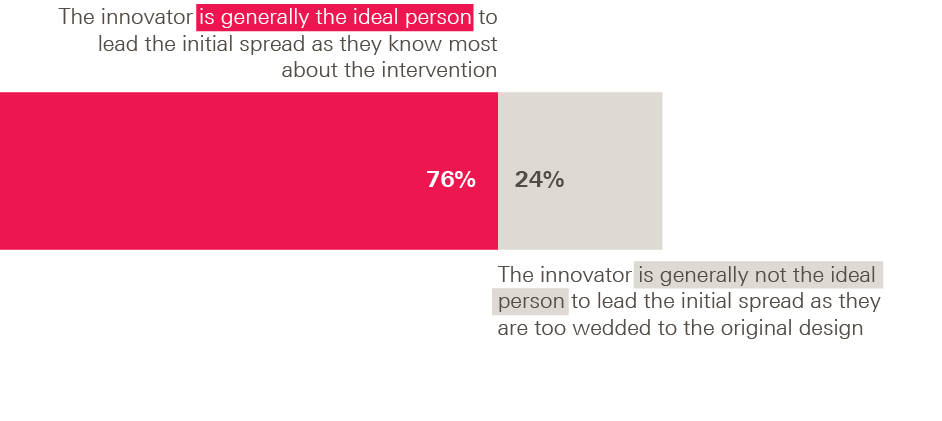
So, although adopters clearly value flexibility and the freedom to adapt the intervention to their own context, it would be wrong to conclude they want as little prescription as possible or an environment where anything goes.
***
One of the benefits of formative and process evaluations is that they can uncover an intervention’s underlying theory of change and throw light on the role played by various contextual factors in facilitating or hindering an intervention’s success, thereby supporting future replication. However, it is clearly also important for innovators, policymakers and programme leaders – not just evaluators and researchers – to be aware of these approaches to conceptualising and describing interventions in order to codify and spread them more effectively. For example, we would echo others who have advocated greater use of programme theory by innovators and improvers in order to enhance intervention description and promote successful replication.
Despite the fact that it contains rich, practical insights for those engaged in spread and adoption, the majority of discussion of these issues tends to reside in the academic literature on evaluation and implementation science, rather than in literature aimed at practitioners. We believe there is a case for making these approaches to codification a standard part of the innovator’s toolkit alongside more familiar skills such as how to make a pitch or how to design a business case. We would therefore encourage those involved in supporting innovators to scale up their work, and those involved in designing courses for innovators, to incorporate these ideas and approaches to conceptualising and describing interventions.
Case study 1
Shared Haemodialysis Care: From a task-focused intervention to a cultural shift
Haemodialysis is a treatment for people whose kidneys are no longer functioning properly. It involves diverting blood through a machine, where it is filtered to remove waste products and excess fluid, before being returned to the body. Easy access to the blood vessels is necessary. If the treatment is undertaken in a hospital setting it is usually required three times a week. The treatment becomes a major part of people’s lives, with each hospital visit lasting up to four hours, and it can sometimes leave patients feeling powerless and entirely dependent on the health professionals caring for them.
A team working out of Sheffield Teaching Hospitals NHS Foundation Trust formalised an intervention called Shared Haemodialysis Care, which gives hospital dialysis patients the opportunity to take a greater role in their own care. Patients are supported to perform any of the 14 standard tasks involved in haemodialysis, such as checking blood pressure, preparing the dialysis machine and inserting needles. Involving haemodialysis patients in their care has been found to empower them and improve motivation, confidence and wellbeing.
From 2016, with funding from a Health Foundation Scaling Up grant, the Sheffield team developed a programme to spread Shared Haemodialysis Care across 12 dialysis units in England, split into two waves of six. This followed a previous initiative that had defined the intervention, as part of the Health Foundation’s earlier programme Closing the Gap through Changing Relationships (2010–2013), which proved essential in providing pilot data and informing plans for the subsequent Scaling Up project.
The Scaling Up project, which ran from 2016 to 2018, was structured as a quality improvement collaborative. It included supporting materials such as a patient competency handbook (to support patients to carry out the standard haemodialysis tasks), posters and leaflets, as well as vehicles for sharing learning such as events, teleconferences, peer networking and an online knowledge-sharing platform. An additional seven sites, including some in Northern Ireland and Scotland, subsequently joined, bringing the total to 19.
The experience of trying to spread the intervention has led those involved to reflect on what Shared Haemodialysis Care actually consists of, and what the essence of the intervention is. As part of the previous Closing the Gap initiative, the programme leaders had specified that ‘doing’ shared haemodialysis required patients to be carrying out at least seven of the 14 haemodialysis tasks (subsequently revised down to five). However, through testing the intervention in multiple sites, the team has discovered that enhanced patient experience is not necessarily achieved by patients carrying out a certain number of tasks, but rather by asking them, ‘What would you like to learn?’ and helping them achieve their goal.
‘Shared care is engaging patients in any aspect of their own care that’s meaningful to them… it’s a concept, really. It’s not a number of tasks that people do.’ Interview with a member of the programme team
This has led the programme leaders to realise that the core of the intervention actually lies in fostering a cultural shift, one where patients and professionals become genuine partners in delivering care, rather than simply teaching patients to perform a certain number of tasks.
‘… When we first started, we thought we were going to find the answer and go, “Oh, bingo”… “This is easy”… It’s much clearer to me now what the essential ingredients are, but it does change and change over time and just recently I’ve… felt, well, this is huge actually, this is a huge culture change, engaging patients in their own care.’ Interview with a member of the programme team
Reflecting the cultural and relational shift that underpins the intervention, the team found that some of the greatest implementation challenges have involved overcoming resistance from staff, who may feel their professional status is threatened, and among patients, who have sometimes been concerned about the security of nurses’ jobs.
So while the tasks remain central to the technical side of Shared Haemodialysis Care and are useful for measurement, the intervention is now conceptualised primarily as a cultural one. In line with this, the intervention is now characterised in a ‘loose’ way, where the emphasis is on achieving an overall goal – supporting patient involvement to a degree that is meaningful to them – and using a capability-building approach to help adopter teams get there. A major focus of the programme is the use of quality improvement methodology, using small tests of change to enable teams to test the intervention and find their own ways forward; teams are supported in improving different aspects of the intervention using PDSA (Plan, Do, Study, Act) cycles, whether that is one of the 14 haemodialysis tasks or aspects of the environment in which care is delivered.
‘… It’s a very loose intervention. I think there’s always a debate about how much you have to know before you start conducting a trial, and how much iteration there can be… There are quite specific ideas around the delivery of care, but [it’s] possibly less specific about how to actually make those changes within a unit.’ Interview with the programme evaluator
And while teams are provided with materials needed to support patients – such as information about the procedures, the patient competency handbook, and so on – programme leaders have encouraged teams to customise these in order to make them suitable to the local setting.
‘… One of the things we learnt… last time, perhaps, was that ‘dissemination by lamination’ wasn’t the thing to do. It wasn’t going to work. This needs to be locally configured by the teams in a way that means something to them, and it would be very different at each site.’ Interview with the programme leader
All of this has meant that, in practice, the intervention looks very different from site to site, though only occasionally has the innovator had concerns about adopters’ interpretations of the original model, such as when one site stipulated that shared haemodialysis could only be performed by patients on a designated ‘shared care’ bed. As a general rule, local flexibility and customisation of the intervention by adopters has been viewed positively by the programme leaders, as both a central aspect of making the intervention work in new settings, and an important source of learning as the intervention spreads further.
‘To just pick up what was happening at Sheffield and say, “Right, this seems to be working at Sheffield. We’re just going to do exactly the same everywhere,” it would be just trying to bang square pegs into round holes all over the country. I think there’d be a lot of resistance. Things clearly wouldn’t work, and they wouldn’t… necessarily be the priorities of the staff. There might be local difficulties which meant that they’d get discouraged.’ Interview with the programme evaluator
‘You’re getting a different perspective on it all the time, aren’t you? I’ve kind of thought that we were all doing similar things, but it turns out everybody has got their own interpretation of it, and learning about and listening to what they’ve got has helped us to understand what the key ingredients actually are, because there were some that were very successful.’ Interview with a member of the programme team
The testing and revision stage
The preceding analysis has highlighted the importance of the context into which an intervention is introduced in determining its success, and thus the need for adopters to be aware of relevant contextual factors when attempting to replicate the intervention.
But it can be hard for innovators to ‘see’ their own context. When something has been achieved successfully in one location it might seem straightforward to document the actions involved, but it may in fact be impossible to know which aspects of context were relevant to the initial success without being able to compare this experience against other counterfactual scenarios. It may therefore be only when an intervention is implemented in new contexts that the comparative information becomes available to enable the innovator to understand what actually made their intervention work first time around.
This is something we often see in Health Foundation programmes. When an intervention that has succeeded in one setting (or group of similar settings) is introduced into a diverse range of sites, the variable fortunes of the intervention can shed light on which intervention components and contextual factors are more or less important for the intervention’s success. Similarly, as adopters shape the intervention to fit their own setting, valuable learning is created about the tolerance of different components to alteration. This gives the innovator fresh insights into what is and isn’t significant for making the intervention work, enabling them to revise the intervention description accordingly. An example of this process, taken from one of the Health Foundation’s improvement programmes, is described in the case study of RIPPLE at the end of this chapter.
What is happening during this initial ‘spread’ phase – a learning process in which the intervention may undergo substantial re-conceptualisation and refinement – often looks quite different from attempts to spread an intervention at later stages of maturity, when it is codified with greater confidence and issues of fidelity are more clear-cut.
Within pharmaceutical and product innovation, such a stage of comparative testing is usually recognised as a formal part of the ‘innovation cycle’ (for example, the ‘field testing’ or ‘beta testing’ stages of product development), but we have found this to be less consistently so with quality improvement and process innovation. Indeed, we often see innovators applying to the Health Foundation with similar proposals and expectations for spread, despite their innovations being at very different stages of maturity.
There may, therefore, be value in recognising this testing and revision phase as a formal part of the innovation cycle, distinct from attempts to spread the intervention at later stages of maturity, as illustrated in Figure 7. An example of this can sometimes be seen in franchising operations, where the early franchisees make a significant contribution to improving the model, identifying and solving problems, and updating the manual; in some cases, franchisors reduce entry fees for initial franchisees because they know the idea is still under development.
Figure 7: Innovation cycle
This concept of a testing and revision stage is supported by our survey of innovators from Health Foundation programmes. They were clearly aware of the possibility of learning from adopters: 91% said they had learned new things about their intervention from adopters’ experiences of implementing it and, of these, 90% said they had changed the way that they describe and communicate the intervention as a result.
Figure 8: Survey of innovators – learning from adopters
Implications for the design of the initial spread phase
Recognising this initial spread phase as a distinct stage of the innovation cycle, one that is primarily about testing and revising the intervention, would enable it to be designed appropriately. There are several considerations here.
First, it would help set realistic expectations for the outcomes of this initial phase, recognising that the main objective is to learn from variations in performance across different sites, rather than assuming that replication will be successful everywhere. This would help avoid artificial pressure to contrive ‘wins’ from every site and instead focus on the learning generated by both positive and negative experiences. Explicitly acknowledging the need to test the intervention in new contexts could also inform the selection of initial adopter sites to ensure appropriate diversity, rather than creating a bias to work only with sites similar to the original (which might be assumed to have greater potential for successful replication).
Innovators’ openness to learning during the initial spread phase emerged in another of our survey questions. When asked to choose between contrasting statements, two thirds (67%) favoured the statement ‘It is better to select as initial adopters a diversity of sites to test where the intervention will and will not work’, with only a third (33%) instead choosing ‘It is better to select as initial adopters those sites where the intervention is most likely to succeed’.
Figure 9: Survey of innovators – initial site selection
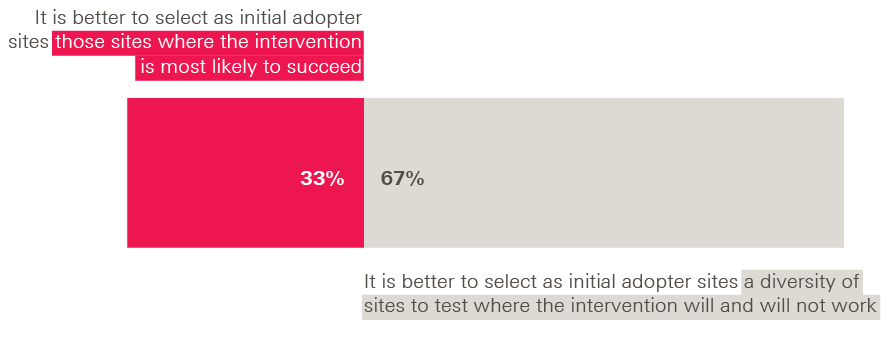
Second, recognising the role of early adopters in generating new knowledge about the intervention has implications for the relationship between the innovator and adopters during this testing phase, where they are cast more as peers engaged in reciprocal learning rather than in the kind of ‘teacher-pupil’ relationship that traditional dissemination models imply. Indeed, we have occasionally seen examples at the Health Foundation where an early adopter makes a greater success of the intervention than the innovator’s original attempt, and the innovator learns a great deal from them. This can sometimes pose a challenge to innovators, who may view themselves as having exclusive knowledge of their intervention, or be attached to aspects of its original form that prove to be superfluous or sub-optimal in new contexts. Innovators therefore need to enter into this phase prepared to revise their own conception of the intervention and to accept that their initial idea will be developed by a wider community. In some cases, where this proves a challenge, innovators may need to bring in others with the detachment and mindset to lead this initial spread phase.
Third, the fact that early adopters are generating new learning that can be used to refine the intervention necessitates mechanisms to capture and share this learning, such as workshops, peer networks and formal evaluation. Here, spread programmes can learn from wider quality improvement initiatives such as improvement collaboratives and clinical communities, many of which invest substantially in creating peer communities to support reciprocal learning. A good example was the Improving Lung Cancer Outcomes Project from the Health Foundation’s Closing the Gap through Clinical Communities programme (2009–2012). Led by the Royal College of Physicians and involving 30 multidisciplinary teams, the project drove a range of improvements in lung cancer treatment, including better access to clinical nurse specialists and shorter referral-to-diagnosis times, through creating a community committed to peer learning. The independent evaluation found that central to its success was a series of peer-to-peer review visits, which enabled sites to learn from each other in a genuinely reciprocal way.,
Such networks don’t necessarily have to be created anew for each new spread programme, though. Box 8 describes one network, the Q community, and the growing role it is playing in the UK in supporting spread and achieving improvement at scale.
In order to better understand whether – and how – interventions work, evaluation is another key part of the picture. Box 9 highlights the work that The Healthcare Improvement Studies Institute is doing in this area to create an evidence base that supports replicable and scalable improvements to health care.
While different structures and mechanisms for sharing learning will be appropriate in different circumstances, the key point is to recognise that the early spread process is often a process of co-innovation – the collective development of an idea through iterative waves of implementation and refinement.
Adopters’ awareness of the learning they generate, and the value they attach to sharing implementation experiences with one another, was reflected in our survey. Nearly all of the adopters who took part in our survey (98%) said that their experiences of implementing the intervention had generated new learning about it. Separately, we asked them whether sharing implementation experiences and learning with other adopters had been a major part of the spread programme and, if so, whether this had been important in helping them to implement the intervention. Of the 91% of adopters who said that sharing learning had been a major part of the programme, a clear majority (58%) said this had been important in helping them (see Figure 10).
Figure 10: Survey of adopters – learning from other adopters
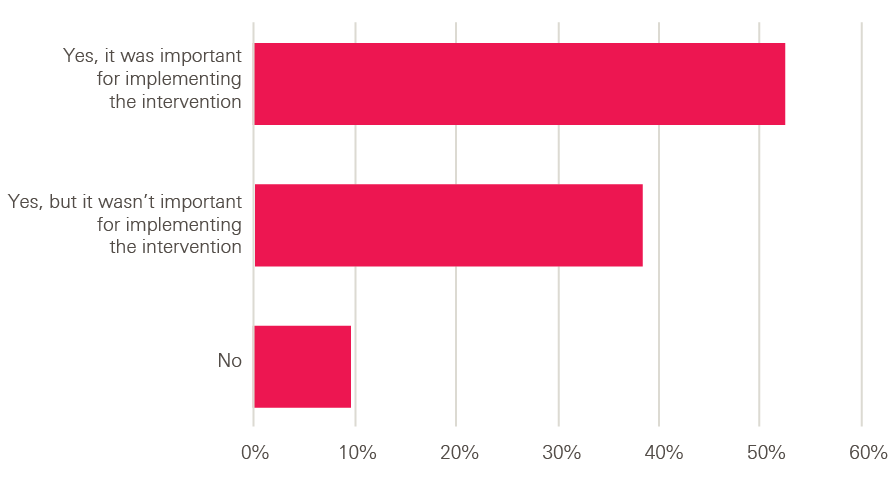
This finding was mirrored in adopters’ responses to a further question, discussed in Chapter 5, about what would have made the biggest difference for supporting adoption; the most popular choice was having more opportunities to share learning with one another.
These survey results speak to the value of nurturing ‘horizontal’ networks of adopters for supporting spread, which can act as a valuable mechanism for sharing learning as well as creating shared vision and values.
Box 8: The Q community
Q is a community connecting people with improvement expertise across the UK. Funded by the Health Foundation and NHS Improvement, Q aims to support people in their improvement work by creating opportunities for them to come together and share their knowledge and ideas.
Formed in 2015, Q is a growing community, currently with over 2,400 members. Its membership is diverse, including those at the front line of health care, patient leaders, managers, researchers, commissioners, policymakers and others – boosting its power as a source of innovation and problem-solving.
Informed by evidence that flexible networks support innovation and spread, Q enables its members to foster productive connections, including by providing opportunities for them to connect face-to-face and online, for example through local and national events and special interest groups (in areas such as primary care or emergency care).
One Q initiative, the Q Lab, develops this approach further by bringing professionals and patients together to make progress on particular challenges over a fixed period of time. Combining social innovation approaches with health care improvement expertise, the Q Lab provides a space for people to work collaboratively on a problem, develop possible solutions, test new ideas and share learning.
Recent evaluations of Q and the Q Lab have found that members are making useful connections they say they would have been unlikely to make otherwise. Members regularly share stories of borrowing ideas and inspiration from others, enabling them to ‘shortcut’ or improve work they are doing locally. This avoids the wasted effort that comes from people trying to solve problems already addressed satisfactorily by others elsewhere.
Box 9: THIS Institute
The Healthcare Improvement Studies Institute (THIS Institute) aims to create a world-leading scientific asset for the NHS by strengthening the evidence base for improving the quality and safety of health care. Co-created by the University of Cambridge and the Health Foundation, the Institute launched in 2018. It is founded on the guiding principle that efforts to improve care should be based on the highest quality evidence.
THIS Institute is boosting research activity to provide more clarity on what works in improving health care, what doesn’t, and why. By evaluating which interventions work in which contexts, and how they work, the Institute aims to create an evidence base that supports replicable and scalable improvements to health care delivery and patient experiences. Through its innovative fellowship programme, the Institute is also boosting research capacity by creating a new generation of highly trained, multidisciplinary experts with skills in researching health care improvement.
THIS Institute’s work is defined by an inclusive approach that combines academic rigour with the real concerns of patients and staff. It engages a broad coalition from the UK’s wealth of expertise across health care, science and beyond, and works closely with multiple partners from different sectors across the UK, as well as patients and health care staff themselves.
Rethinking the pipeline model
Recognising the role of early adopters as ‘knowledge generators’ challenges the traditional ‘pipeline’ model of innovation, which sees knowledge as generated by the innovator and merely transmitted to others through the diffusion process. As Miller and Shinn put it in the context of health promotion programmes, under a more sophisticated view of innovation and adoption, ‘dissemination becomes not simply the routine application of knowledge developed elsewhere and codified… but the theoretically motivated search for underlying principles of programmes or practices that can inform both understanding of change and programmes to create it’.
This perspective expands on the traditional set of ‘adopter categories’ popularised by Everett Rogers in the 1960s: innovators, early adopters, early majority, late majority and laggards. These were conceived as population types, based on their likelihood of exhibiting certain behaviours, but these categories can also be applied to the diffusion and adoption of a single innovation. In this context, ‘innovators’ are, according to Rogers, the initial adopters of the innovation, early adopters are the next set of people to adopt the idea, and so on, with laggards being the last (see Figure 11).
Models of diffusion rooted in a ‘commercialisation’ paradigm have tended to draw a sharper distinction between pre-market ‘innovation’ stages and subsequent commercial ‘adoption’ stages than is the reality in much process innovation and quality improvement in health care – and, indeed, in other sectors too like software, where developers can iteratively update their product. (Note that the description of adopters as knowledge generators here is different from the notion of ‘lead users’ as innovators, in which the idea and pressure for innovation comes from a group of users, but which is nevertheless still conceived as a ‘pre-diffusion’ stage of the innovation cycle.)
As Figure 11 illustrates, a realistic model of diffusion in health care requires a far more blurred boundary between the idea of the innovator and the initial adopter/early adopter categories. Here we agree with Hawe, who argues that conventional terminology is unhelpful because ‘it privileges a pipeline metaphor of knowledge generation at the expense of understanding and finding ways to convey primary knowledge emanating from practice contexts’ and that ‘a new language is needed that gives expression and legitimacy to models of co-production of knowledge’.
Figure 11: The initial spread process as co-innovation
Case study 2
RIPPLE: How implementation in new contexts can build deeper understanding of the intervention
Chronic obstructive pulmonary disease (COPD) is the second most common cause of emergency admissions in the UK and causes one in 20 deaths. Its symptoms, such as breathlessness, can lead to and amplify anxiety, low self-esteem and social isolation, which in turn can affect mental health and result in poor self-management and lack of engagement with key treatments. In a British Lung Foundation survey, 90% of people with COPD said they were unable to participate in socially important activities.
The RIPPLE clinic (Respiratory Innovation: Prompting Positive Life Experience) was developed in Coventry with the support of a Health Foundation innovation grant in 2014. It is based on the hypothesis that outcomes for people with COPD can be improved by addressing factors such as social isolation, depression and anxiety, rather than simply improving their lung function. In this sense, it is primarily a social, rather than a clinical, intervention. The idea was to take consultations out of a clinical setting and combine them with social activities, such as yoga and bingo, in a way that would reduce isolation for people with severe COPD. The intervention uses a community space to host sessions, given that clinical settings are not always the best environment for engaging patients and facilitating discussions. The session also provides opportunities for people to take part in education and rehabilitation activities. Attendees have reported an increased ability to self-manage compared to before they attended.,
‘For me, the more I’ve got into it, the more I think it’s a huge philosophical change in the way you deal with people with chronic lung disease, actually… I would say for about 95% of the people who attend the group regularly, it’s actually, “This is the only time I get out of the house this week”.’ Interview with innovator
In 2015, the RIPPLE team received a second grant from the Health Foundation, as part of its Spreading Improvement programme, to spread the model to a further six communities in the East and West Midlands. The new sites were given funding, support and advice, as well as opportunities to share their knowledge and experiences.
RIPPLE is a complex intervention, consisting of multiple components. The initial demonstration project had enabled the programme leaders (including the innovator) to begin to identify its core components. These subsequently became framed as a set of ‘pillars’ of the clinic model – for example, activities to reduce social inclusion, transport provision, third sector involvement, exercise activities, mental health support, and so on – and were set out in the ‘Expression of Interest’ document seeking proposals from teams to be part of the Spreading Improvement project.
‘We were really clear that the delivery venue had to be a normal part of a community, and not an NHS building. They had to think about transport as well, because getting people… there easily is one of the big things with social inclusion… We also made sure… that the third sector were… part of the process. So more often than not, it’s the third sector that actually deliver and run these projects, because they know local communities.’ Interview with programme leader
Many aspects of context potentially add to RIPPLE’s complexity. For example, RIPPLE is co-produced with patients, so each clinic’s social activities naturally evolve around patient preferences. Implementation is also affected by the distinct transport requirements and recruitment challenges faced by urban and rural communities. The quality of relationships with other services may also be important, as the intervention brings together partners from organisations with contrasting working cultures and practices. According to the final evaluation report, the model is ‘complex, with no one-size-fits-all [approach] to improving COPD care across varied sites and locations’.
In line with this, the description of the intervention produced by the innovator and programme leader for the ‘Expression of Interest’ document embodied a loosening approach, focusing on the underpinning principles (the ‘pillars’) and goals of the intervention, without prescribing how clinics should be set up. This has given adopter teams a sense of ownership of the intervention, enabling them to adapt it to suit their local context and population group.
‘We wanted to very much co-produce with people with COPD and have that central in the design and implementation, and they [the programme leaders] allowed us to do that and really kind of gave [us] the power.’ Interview with implementation site lead
‘It [the Expression of Interest document] had something briefly about the outcomes but it doesn’t give you any detail about the clinic… I have to say we were kind of given free rein to go from there. As far as policies and anything written, I would say minimal.’ Interview with implementation site lead
‘Self-management… and tackling social isolation were also the key principles, but [it] was loose in terms of how you deliver that.’ Interview with implementation site lead
‘I think you have to go with what people are willing to do locally. It’s very difficult to impose a model on other people because you have to have their engagement and ownership.’ Interview with innovator
The adopter sites have made a range of adaptations to the model. For instance, one substituted the input from a hospital consultant with that of a respiratory specialist, physiotherapists and nurses, because it was economically unviable for a consultant to travel to the clinic. Another added hospice involvement to the model – ‘because actually that was important as far as the COPD patients that we look after’ – as well as input from the fire service to perform safety checks for people going home with oxygen.
The variations and adaptations made to the clinic model during this spread phase are generating useful lessons, improving the innovator’s understanding of the degree to which the intervention can be modified. For example, there have been significant variations in terms of who sets up the clinic: the Coventry clinic was set up by a secondary care physician, but the clinics at the adopter sites have been set up by a range of organisations, including clinical commissioning groups (with clinician input), general practices and third sector organisations. The primary care-led clinic in particular challenged the original model, since patients could self-refer in a way that hadn’t been tested before. The innovator acknowledged that this model ‘caused us both some concern to start with’, but it has since proved highly successful, with preliminary data suggesting that unplanned hospital admissions have been reduced for those attending.
‘I think we have learned from other sites. I like to think of the project as an ongoing, organic thing that grows and changes.’ Interview with innovator
‘… They seem to be very interested in people doing it slightly differently, because again, that always kind of informs potentially how this could be spread further.’ Interview with implementation site lead
The spread phase has also enabled the programme leaders to clarify when variations have departed too far from the original theory of change – for example, when one team wanted to replace an in-person clinic with a virtual clinic.
‘… They thought they could have a sort of Facebook community rather than a real meeting and we had to be quite firm with them about that and [say] there isn’t really any medical input into their model.’ Interview with innovator
More generally, adopter teams have provided valuable learning for the programme leaders by identifying new solutions to particular implementation challenges, as when one site managed to overcome problems accessing a collaborative IT platform.
‘We now know the way round it, we now know the things that if people say “We can’t do that,” we can actually show them, “Well, yes you can”, because it’s been done.’ Interview with programme leader
During the course of the programme, the innovator and programme leader have also begun to learn more about the respective importance of each of the pillars. For example, while the social inclusion pillar remains central to the intervention, they have become less convinced that the physical exercise pillar is as important for the effectiveness of the clinic, based on the experiences of the adopter sites. These experiences have also reaffirmed that hosting the clinics in community settings rather than clinical settings is core to the intervention, but within this constraint they have learned that the clinics can work well in a variety of different settings, from church halls to football grounds.
This Spreading Improvement project concluded in 2018, and the independent evaluation has reported reduced social isolation and some observed improvements in health status for participants. Building on the experiences of the adopter sites, the innovator believes that the next phase of spread could be more specific about how the clinics could be set up, perhaps in the form of a menu of options based on what others have done.
‘I think we could probably produce a handbook now on how to do it. With a sort of menu, so you have some suggestions. You could probably give two or three suggestions under each section, couldn’t you? There would sort of be the main recipe, and then some variations.’ Interview with innovator
Designing spread programmes
The analysis in previous chapters suggests that adopting a complex intervention is hard work, and that adopters play a very significant role in translating an intervention into a new setting successfully. In this chapter, we look at two consequences for the design of spread programmes: the need to build adopters’ commitment to implementing a new idea and the need to support them in doing so.
Building adopter commitment
The significant role adopters play in adapting complex interventions suggests that attempts to replicate interventions at scale will be more likely to succeed if they recognise and respect the centrality of adopters’ agency in this process. Most obviously, it matters that adopters want to implement a new idea and are committed to doing so, particularly with complex interventions that may necessitate behaviour change from those involved. Nevertheless, this point is often overlooked within national programmes (particularly in the public sector, where providers can be subject to central direction), which tend to focus on the supply of new ideas to the health care system rather than on generating a desire among potential adopters to adopt them.
Specifically, building a commitment to implementing a new idea requires both: (i) acceptance of the problem and the proposed solution (that is, a willingness to implement it in principle);, and (ii) the motivation to put the solution into practice.
How best to gain acceptance and generate motivation therefore become crucial matters for programme design. Achieving consensus on the problem and the proposed solution will usually require engaging adopters in what is being proposed, and may well benefit from giving them the chance to input into or co-design the solution. Similarly, ensuring that adopters are motivated and remain so over time may also require engaging them in shaping the solution and giving them the autonomy to take ownership of it.
These issues are crystallised most starkly when programmes are mandatory or have a strong element of top-down pressure – for while mandating participation might ensure that organisations join a programme, it does not by itself achieve acceptance of a new intervention or the motivation to implement it. But these issues remain a challenge even when participation in a programme is voluntary. A review of evaluations of Health Foundation improvement programmes between 2003 and 2011 concluded that ‘improvement interventions are often “essentially contested”: everyone may agree on the need for good quality, but not on what defines quality or how it should be achieved’. Failure to achieve consensus can hinder uptake and effective implementation, as Box 10 illustrates.
Box 10: Valuing the adopter’s perspective
The Health Foundation’s Safer Clinical Systems programme (2008–2016) sought to improve patient safety by developing the capacity of teams to diagnose system-level weaknesses and introduce interventions to address them. A prominent feature of the programme was its goal of changing the way organisations approached safety from the prevailing reactive, incident-based approach to a more proactive, risk-based one.
One hospital-based team targeted the problem of unplanned readmissions from care homes. The team saw the underlying causes as poor communication between the hospital and the care homes, and inadequate community-based support. Several interventions were introduced to address these problems, including a community geriatric team, a 24-hour telephone support service and an information form to accompany transferred patients. Yet the care home workers believed a more important issue was that patients were sometimes being discharged when it was neither appropriate nor safe to do so – for example, at weekends without the necessary medication or equipment. As a result, they didn’t fully accept that the interventions introduced were the optimal ones for addressing the problem, and resultant tensions in the goals and priorities of the hospital team and care home teams ended up frustrating the improvement work.
This case illustrates the importance of considering the adopter’s perspective from the outset. According to the evaluators, in this case the care homes held valuable insights into the nature of the problem, which could have led to the development of better interventions had they been taken into account earlier.
Considering the adopter’s perspective seems especially crucial when working across organisational boundaries. A recent evaluation by the Improvement Analytics Unit (a partnership between NHS England and the Health Foundation) of providing enhanced support for older people in care homes in Rushcliffe found that residents experienced reduced A&E attendances and admissions compared to a matched comparison group. The evaluation notes that the providers involved had a programme of work specifically to build relationships across organisational boundaries and engage care home teams and that ‘It is possible that this has led to greater common understanding of the nature of the problems that need to be addressed and, therefore, more effective interventions’.
Generating acceptance of the need for change and the motivation to change can require far more than the presentation of evidence. Behavioural science suggests these phenomena have strong psychological and social dynamics – being influenced by attitudes, norms and relationships. Box 11 describes three important areas where behavioural insights can inform the design of spread programmes in this respect: peer leadership, peer communities and adopter ownership.
Box 11: Behavioural insights and spread
When it comes to adopting new ideas, behavioural science suggests that steps such as accepting the need for change and then moving from intentions to actual behavioural change have important psychological and social dimensions. Here we look at three such issues that relate to specific aspects of the design of spread programmes.
Peer leadership. The source of any change message is a crucial factor in building the case for change. Evidence shows people are more likely to listen to and be influenced by others like them – whether in terms of identity, background or behaviours – particularly when the topic is related to the group identity of the ‘messenger’ and ‘receiver’. (This can sometimes be a rational strategy as it means the messenger may be more likely to understand the specific issues faced by the receiver.)
This phenomenon can be particularly significant in health care, given the demarcation of professional identities and the strong role that professional bodies tend to play in determining values and behaviour. For this reason, professional and peer leadership can be especially important in building a case for change and developing consensus around a solution. As a recent Health Foundation report put it: ‘Active work to secure credibility is needed. This is likely to imply working with a variety of professional groups on their own terms, and aligning the project with each group’s values and notions of best practice’.
Peer communities. Social networks can play an important role in generating a commitment to change as they not only transmit information but also shape norms and values, which can be powerful drivers of behaviour and of the adoption of new ideas. Thus, creating a peer community or network of adopters can play an important role in generating and maintaining a collective commitment to change (as well as in supporting the kind of peer-to-peer learning discussed in the previous chapter).
Studies suggest that adoption is influenced by network strength, which is determined by factors such as frequency of contact, geographical proximity and degree of intimacy between the ‘champion’ and the adopter. For example, one study found that primary care practices were more likely to be early adopters of a new drug if neighbouring practices had already done so. Spread programmes could therefore benefit from fostering the kinds of horizontal structures that are commonly used in wider quality improvement work, such as clinical communities and improvement collaboratives.,
Ownership of the intervention. The degree of ownership of an intervention among adopters – reflecting their role in helping to create and shape it – may also be important for building and sustaining commitment. This goes beyond the need to adapt an intervention simply in order to make it work in a new context; research suggests that the very act of creating something can be important in generating attachment to it. This has been named the ‘IKEA effect’, based on the observation that people value products they have made themselves more highly than identical, externally-assembled versions.
A range of psychological mechanisms may underlie this phenomenon, including the increase in attachment that can occur with effort, the positive feelings of efficacy that accompany the successful completion of tasks, and potentially also the inherent enjoyment of the creative task itself. Norton et al. suggest that these psychological mechanisms also apply to organisational change and may contribute to the so-called ‘not invented here’ syndrome, whereby organisations reject good ideas developed elsewhere in favour of their own internally developed ideas. They also suggest that these psychological mechanisms may not be susceptible to influence. In any case, this highlights the utility adopters gain from being involved in the process of creating and shaping an intervention, and thus the potential advantages of allowing appropriate autonomy and ownership of the intervention for helping to generate a commitment to implementing and sustaining it.
The innovators from the Health Foundation’s programmes who took part in our survey clearly recognised the need for adopter autonomy and ownership. When asked whether innovator control or adopter autonomy should be prioritised in supporting spread, the vast majority of innovators (86%) chose the statement ‘It is better for the innovator to allow adopter autonomy to encourage local ownership’, with only one in seven (14%) choosing ‘It is better for the innovator/programme leader to try to control adopter implementation in order to ensure fidelity’.
Figure 12: Survey of innovators – control versus autonomy
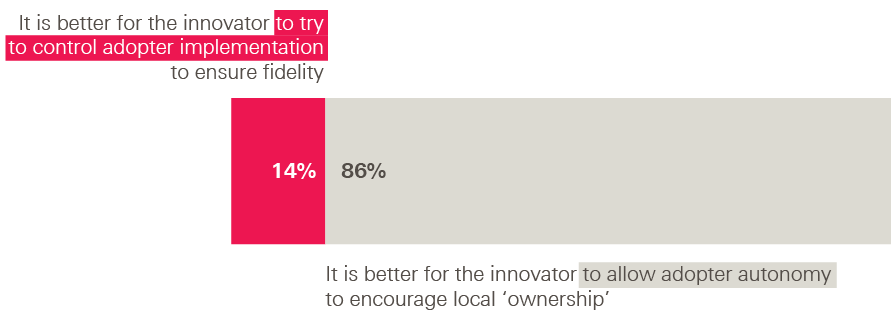
That factors such as peer leadership, peer communities and adopter ownership matter is also clear from a history of programmes that have underperformed because they didn’t pay enough attention to getting buy-in from everyone concerned or creating a community of adopters to support the spread process. One such example, the Matching Michigan programme, is described in Box 12.
Box 12: Matching Michigan
Recent research by Dixon-Woods and colleagues, supported by the Health Foundation, looked at the differing fortunes of two programmes to reduce central venous catheter bloodstream infections (CVC-BSIs)., One was the Michigan Keystone programme (2003–2006), which successfully reduced CVC-BSIs in intensive care units (ICUs) across Michigan. The other was an NHS programme in England called ‘Matching Michigan’ (2009–2011), which sought to reproduce the success of the Keystone programme, but failed to have an impact over and above the contemporary background trend.
One key difference between the programmes was professional leadership. The Michigan programme was voluntary and led by a state hospital association and a university. They deployed ICU ‘insiders’ to promote the programme, with whom participants could identify. The researchers found that this was essential for establishing trust, securing legitimacy and influencing professional norms: ‘the credibility and legitimacy of the evidence and the proposed action had to be established through social processes…The authority of evidence does not stand on its own but requires support from the moral authority of those seeking to deploy it’. By contrast, the Matching Michigan programme in England was led by a government agency and followed a series of other initiatives to tackle CVC-BSIs that had been perceived by some as ‘top-down’ and punitive. This undermined engagement and made it difficult to persuade participants that the programme was necessary. According to the researchers, ‘The location of the programme in a government agency rather than a professional organisation or research collaboration appeared to contribute to an alienating sense of distance on the part of some front-line clinicians. Matching Michigan was seen as imposed from the outside and lacking in professional ownership’. The researchers concluded that these differences profoundly affected the English programme’s prospects, since there were difficulties in persuading people of the problem (infection rates were in fact much lower in England at the start of the programme than they had been in Michigan) and also that the programme was the solution (best practices were already being adopted, and the programme evaluation showed a strong secular trend towards improvement).
Horizontal relationships were another key mechanism underpinning the Michigan programme’s success. By bringing participants together in workshops, the programme built a networked community, which helped create shared norms and generate commitment and ownership among participating teams. That is not to say there were no top-down elements to the programme; there was a vertical structure that provided leadership and coordination and made judicious use of top-down pressures, such as the deployment of data for benchmarking. But the researchers found that these vertical forces were balanced by strong horizontal ones: ‘By developing horizontal links between the participating units, the programme was able to mobilize social forces beyond what would have been possible had the model been solely vertical’. The English programme, by contrast, did not invest in creating such horizontal links. This meant that participating units lacked the experience of being part of a collaborative community working together towards shared goals, which in turn reduced the possibility for influencing professional norms. Furthermore, because the English programme was mandatory, it inspired varying levels of commitment and ownership: ‘Whereas Michigan generated emotional commitment, ICU staff in England did not feel the same affection, identification and ownership for the programme’.
Supporting adopters to implement new ideas
In addition to adopter commitment, successful spread will also clearly rely on adopters’ ability to implement the intervention in question, that is, on their readiness and capability to do so, as well as on them having sufficient opportunity to do so. So adoption may require time, resources and organisational capacity.
While this may seem obvious, it is worth emphasising because spread programmes have often been designed without considering these factors. For example, in programmes that seek to pilot and then ‘roll out’ interventions, there can be an assumption that once an intervention has been successfully piloted, the hard work has been done and spreading the intervention will be straightforward. This can also be accompanied by an assumption that adoption can happen quickly, even though the innovator may have taken years to develop and refine the intervention within their own organisation. To take an example, a recent Health Foundation study of progress made by the New Care Models vanguard sites in England found that work had already been going on in these sites for between two and ten years before the New Care Models programme started.
But as we have seen, adopting a complex intervention may itself necessitate substantial creative effort and reinvention. In some cases, it could take as long as the initial development of the intervention, perhaps even longer if there needs to be an initial period in which the adopter site has to develop the readiness and capability to implement the intervention that the innovator site had already possessed at the outset of their work.
These traditional assumptions about innovation and spread are also often reflected in the distribution of resources within spread programmes, which tend to focus funding on demonstration projects – pilots, ‘vanguards’, ‘pioneers’, and so on – and then expect everyone else to follow with little, if any, financial or organisational support. Again, the experience of the recent New Care Models programme is instructive. The National Audit Office report on the programme found that NHS England provided a total of £329m to the 50 New Care Models vanguards for testing their proposed new care models, along with a further £60m to accelerate implementation and maximise opportunities for replicating them. But it also highlighted that, despite an ambition that 50% of the population would be covered by these new care models by 2020–2021, details of how the new models were to be spread were not set out, and further resources that might have supported spread were instead reallocated to reducing trusts’ financial deficits.
The logic of the discussion here, however, is that resources may instead need to be invested in building adopters’ readiness and capability and allowing them to have sufficient time and space to do the hard work of translating the intervention into their own context.
All of this has consequences for how spread programmes are designed. Most obviously, programmes may need to include support for implementation. This could involve funding to help an adopter site create the necessary capacity (for example, to establish project-management teams or backfill staff positions) or to support the upfront costs of adoption (for example, for initial ‘double running’ costs). It could also include embedded support in areas like analytics and evaluation, or mechanisms to support peer-to-peer or organisation-to-organisation learning.
Successful adoption may also require a decent chunk of time and ‘headspace’. This includes building in sufficient time for adopters’ journeys to a state of readiness and allowing for a proper set-up phase prior to implementation. While ambitious timetables can help to motivate faster implementation, it can be counterproductive to impose unrealistic timescales for change, which can divert attention towards finding quick wins and away from the hard work of diagnosing underlying problems and designing necessary changes.
Our survey of innovators and adopters provides some useful data on these issues.When innovators were asked which changes, with hindsight, would have made the biggest difference for helping adopters to implement the intervention, the option of providing more training and support to build adopter skills and capabilities ranked highly among the options given (chosen by 48% of innovators), second only to doing more in advance to support adopter readiness (57%).
Figure 13: Survey of innovators – what would have helped most?
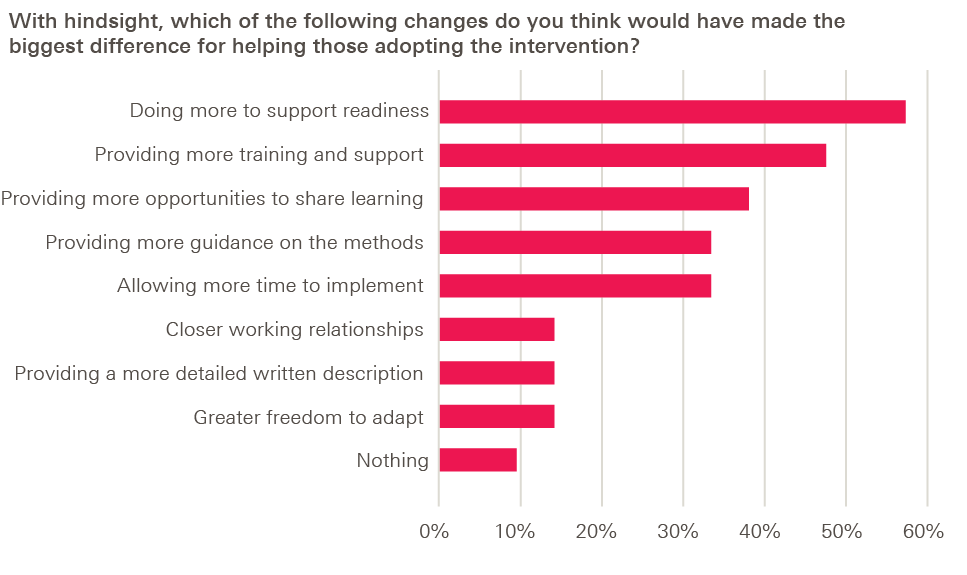
These priorities were also reflected in adopters’ responses to the same question. For adopters, the most popular option was greater opportunities to share learning and experiences with one another (chosen by 41% of adopters), with supporting adopter readiness ranked second (31%) and providing more training and support ranked equal third (26%), along with providing more time for implementation.
Figure 14: Survey of adopters – what would have helped most?
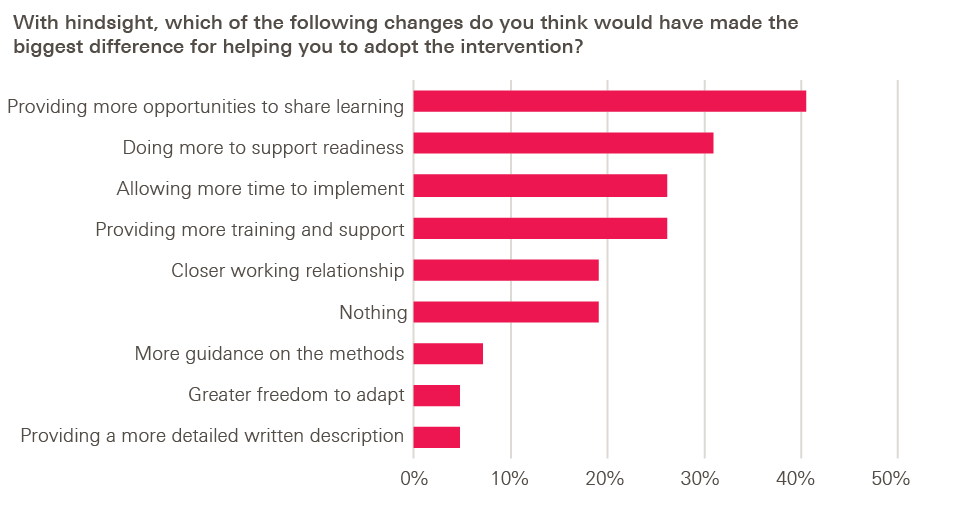
In summary, spread programmes should aim to build and maintain adopters’ commitment, including seeking consensus on the problem being tackled and the proposed solution, as well as allowing appropriate autonomy and encouraging adopter ownership of the intervention. Spread programmes also need to provide adopters with sufficient support for implementation, and this may have implications for the balance of investment between supporting innovators and adopters.
Case study 3
Situational Awareness for Everyone (SAFE): How adopter communities can help drive spread and create a determination to succeed
Certain outcomes for acutely sick children in the UK are significantly worse than in other countries. The causes are complex, but include frequent failure to recognise severity of illness, inappropriate response to deterioration and poor communication.
Originally developed in the military, ‘situational awareness’ has recently gained interest within health care. It seeks to support the anticipation of potential problems through improved consciousness of, and attentiveness to, the environment. Huddles – rapid exchanges of key information among the staff involved in a patient’s care – are one way to operationalise situational awareness. They typically last between five and ten minutes, involve staff from different professional groups, and follow a set of scripted questions. By encouraging information sharing, they help health care workers spot when a patient’s condition is deteriorating. Studies have shown that huddles can improve patient safety, enhance collective awareness of harm, improve collegiality among teams and increase clinical accountability.
In 2012, a team at Great Ormond Street Hospital for Children (GOSH) piloted huddles based on a version of this idea developed at Cincinnati Children’s Hospital in 2008, where staff had reported that the huddles played a significant role in highlighting safety problems and identifying clinical deterioration. Following this, from 2014 to 2017, as part of the Health Foundation’s programme Closing the Gap in Patient Safety, the Royal College of Paediatrics and Child Health (RCPCH) worked with the GOSH team to support the implementation of huddles in paediatric units across 12 hospitals in England, divided into two waves of six.
The SAFE programme approached the implementation of huddles more flexibly than in Cincinnati, using PDSA cycles to guide the form the huddle should take to deliver the best results locally. Accordingly, the intervention was loosely defined at the outset and the programme featured a strong element of testing. Knowing it was going to be implemented in a range of contexts, the programme leaders set out the principles underpinning the intervention and provided resources to aid implementation, such as the SBAR (Situation, Background, Assessment, Recommendation) communication tool.
‘… There are many different ways you can apply the principles to create huddles.’ Interview with the programme leader
‘Well, I think particularly with the DGHs [district general hospitals], it was hard for them, because they didn’t have anything to really model it on. I mean, when we started it, there wasn’t anybody else doing it, so we didn’t feel like we had to do it in an absolute, particular way.’
Interview with an implementation site lead
The programme leaders did not prescribe how teams should implement huddles; teams were sent articles based on Cincinnati’s experience, but there was no handbook and no protocol for documenting the huddle. The main way in which teams learned about the intervention was at the initial programme learning events, with a presentation from the programme leader on elements such as the questions to ask in a huddle.
These learning events subsequently became a valuable opportunity for teams to exchange ideas, insights and experiences. Teams would share what they were doing and what new things they had tried. Through the events, the programme leaders created a strong network of adopter sites with the dynamic of a ‘community of practice’.
There were differing fortunes among the initial wave of sites – some did well, while others struggled – but valuable learning emerged from all sites. For example, the programme leaders gained insights into how the intervention could be implemented in a district general hospital, given that it had previously been used only in a tertiary setting.
‘Certainly the idea was that the first 12 sites would sort of try different things, try things out and you’d learn what the errors were and what the pitfalls were so that they could take it to the other teams… what they took to the wave two and three teams was things that had come up that looked like they could be used in lots of different areas, and not just in a tertiary hospital, because I think that was the concern… how is it going to sit with the DGH? But it was good how it was six children’s hospitals and then six DGHs, so you could see how some things worked better in one area compared to another.’ Interview with an implementation site lead
Several adopter sites started to adapt the huddle to suit their own context, and in ways not originally envisaged by the programme leaders. For instance, one site created what it called a ‘druggle’ – a huddle on a neonatal ward focused on medication errors, with representation from the pharmacy team; another site developed hospital-wide situational awareness rounds, where the bed manager collected information about patients’ conditions, staffing levels and bed occupancy rates in order to find the most appropriate wards for new admissions. These kinds of adaptations illustrated the extent to which the intervention could be successfully modified while still retaining fidelity to the key underpinning principle – to support the rapid and effective communication of information across multiple professional groups. The initial phase also demonstrated a number of benefits of the huddle that went beyond identifying patients at risk, including improving team working, role-modelling appropriate behaviours, and breaking down professional barriers.
‘… Just bringing the team together, just somehow physically bringing them together, once a day, even just for three minutes, did improve team working, in a way that we hadn’t expected it would. So, it sort of broke down all these barriers of doctors and nurses, and we’re just one team looking after the patient. It was very powerful… There was a huge amount of role modelling.’ Interview with an implementation site lead
The experiences of adopter sites and insights from the programme evaluation shed light on the elements that seem to be core to the intervention – it has to be multidisciplinary, last no more than 10 minutes, each site needs to use their own huddle script consistently, and the huddles require a ‘champion’ – as well as those that are peripheral.,
‘I don’t think it matters what time it’s done. I don’t think it matters really where it’s done, as long as you’ve got the patient list in front of you. I don’t think it matters what you call it… as long as you know it’s a safety briefing.’ Interview with an implementation site lead
‘… It takes a champion… somebody who is there to make sure everyone else is on board and that it’s on track and that they’re invested and they think it’s important.’ Interview with the programme evaluator
The learning from this period of testing has generated a number of resources to support wider implementation of huddles; for example, a number of teams developed huddle scripts during the programme.,,,
‘I think they got much clearer on the model through that initial process and in a sense were looking for those first 12 sites to help them shape what the model was… I think the first sites were very much the pioneers and they sort of galvanised the approach and, yes, then it was a case of rolling it out as a more clearly articulated programme.’ Interview with the programme evaluator
Over time, the community of adopters has grown. During the course of the programme, significant interest was generated among further prospective sites by local and national media coverage and through events and dissemination activities coordinated by the RCPCH. This led to 16 more sites joining as part of a third wave. According to the evaluator, the first- and second-wave sites were perceived by those not yet participating as pioneers of something that was exciting and offered real benefits.
‘I think there was something about perceiving this group of 12 to be in the sort of inner sanctum, trying this new, exciting thing that had real face validity… there was a combination of it being a sort of elusive thing that they [the third wave sites] weren’t involved in yet and also, yes, the passion and just the common sense of it really, I think.’ Interview with the programme evaluator
This meant that when the third wave of sites came on board there was an air of healthy competition; they were not only able to hit the ground running, benefitting from the experience of the first- and second-wave sites, but also had a real determination to succeed.
‘… They were much more enthused and determined to… get everything embedded quicker and they put that down to various things, I recall. I think [it] was this idea of being the ones that didn’t get it in the first place. There was almost an air of competition or just wanting to prove that they could do it well, and where other people were already out there doing it, and they wanted to get up to speed.’ Interview with the programme evaluator
As interest in the programme grows, a fourth wave is now beginning. This will see the 28 implementation sites from the first three waves ‘buddy’ with a neighbouring organisation to support them in introducing huddles. A total of 50 sites have now completed the programme.
Conclusion: Putting adopters front and centre
In this report we have explored a range of challenges involved in spreading health care interventions that arise specifically from the complex nature of such interventions. These challenges have encompassed issues with codifying interventions, with capturing and mobilising the knowledge generated as an intervention is implemented in new contexts, and with designing spread programmes.
In doing so, we have highlighted the importance of codifying interventions in ways that reflect their social, context-sensitive and dynamic nature and argued that innovators should be exposed to the theoretical approaches for handling complexity that exist in the academic literature. We have also emphasised the importance of a discrete testing and revision phase in the innovation cycle for process innovations and quality improvement initiatives. Finally, we have highlighted some consequences for the design of spread programmes, including the appropriate balance between horizontal and vertical forces and the degree of support required for adopters to translate ideas into their own context successfully.
Recognising the role adopters play
Chapter 2 highlighted the context sensitivity of complex interventions and the need to adapt interventions to new contexts. It also highlighted the dynamic and evolving nature of such interventions and the responsibility this places on the adopter to navigate issues as they arise.
These observations underline the crucial contribution adopters make in the successful spread of new ideas. They highlight that adoption is often hard work, and that even when an idea has been successfully piloted, it may still require a substantial degree of fresh effort and creativity to make the idea work in a new setting. They also suggest the potentially important role adopters can play in generating new learning about an innovation, as they adapt it to fit new contexts and try out new things – learning that can help to refine and improve the innovation as it spreads.
We emphasise these points because to some extent they cut against the grain of traditional thinking about innovation and spread. They challenge conventional notions of the division of labour between innovator and adopter and the assumption that once an idea has been successfully demonstrated, the hard work is over. They also challenge the traditional ‘pipeline’ model of innovation, which sees new knowledge as generated by the innovator and then transmitted to others through the diffusion process. On both of these issues, the research presented here suggests that the process of innovation and diffusion in health care is often a much more distributed effort.
Implications for policymakers and programme leaders
Recognising the crucial role adopters play in successful spread has important implications for policymakers and those designing and leading spread programmes. This includes those overseeing local programmes – such as commissioners, AHSNs, regional and national improvement bodies or professional networks – as well as system leaders overseeing national change programmes.
First, before initiating large-scale spread initiatives, it is important to establish whether the intervention has undergone comparative testing to identify its core features and determine their tolerance to variation, and also whether it has been codified in a way that will help adopters to understand the relevant social, cultural and other contextual factors and adapt it to their own setting. If not, the initial spread phase will need to create opportunities to test the intervention across a suitable range of sites and contexts, and then to refine it and revise the intervention description accordingly. This will require mechanisms for capturing and absorbing the learning generated, such as peer networks and process evaluation.
Second, spread programmes should aim to build and sustain adopters’ commitment. This includes seeking consensus on the problem being tackled and the proposed solution, as well as using mechanisms such as peer leadership and social networks, where appropriate, to influence attitudes and norms. Also important is striking the right balance between ensuring fidelity to the original design and allowing appropriate adaptation – both to ensure the intervention can work in different contexts and also to encourage adopter ownership more generally.
Third, spread programmes need to be designed in ways that better support the task of adoption, including building adopter capability and readiness, providing support for implementation and giving adopters enough time and space for their work to bear fruit. Chapter 5 suggested what this might mean in practice. In summary:
- training and assistance for teams to build the capabilities needed for successful implementation, recognising that the intervention itself is rarely a magic bullet, but needs to be surrounded by the right skills, behaviours and culture to work properly
- funding for adopters to support the costs of project management, such as establishing a project management team and backfilling staff positions
- funding for adopters to support the upfront costs of implementation, such as initial ‘double running’ costs where a new service or way of delivering care has to be trialled alongside the existing one (even where a new intervention can save resources, it may take time for these savings to materialise)
- assistance with data analytics and evaluation, so adopters can understand the impact of the changes being made and identify relevant implementation issues
- support for peer networks and other mechanisms to capture and share learning among adopters so they can learn from each other’s implementation experiences
- ensuring that adopter teams have sufficient time to develop their capability and readiness and to implement and refine the intervention, recognising that it takes time for any improvement to demonstrate impact, and that even the most effective projects encounter obstacles and setbacks at some point.
Measures such as these may well require shifting the balance of investment between supporting innovators and adopters; it is usually tilted in favour of the former. But better funding of adopters would not only improve the chances of successful replication; it might also help stimulate a ‘demand pull’ from provider organisations, encouraging them to adopt promising new ideas.
Beyond the design of specific spread programmes, policymakers and system leaders can also ensure that health care providers are better equipped for, and there are more receptive contexts for, adoption more generally by supporting organisations to build their improvement capability. This will require investment in the basic human and technological infrastructure of the NHS, for example:
- Provider management and leadership quality is associated with the adoption of best practice. Particularly important is the role of management and leadership in influencing organisational culture: a ‘learning culture’, where staff feel safe to question existing practice, can be a key enabler of innovation and improvement, while a risk-averse, blame-focused culture is a huge barrier. Also important is a willingness to look externally and bring in knowledge from other organisations. Measures to support health care providers with leadership development could therefore make an important contribution to improving the landscape for spread and adoption.
- Provider organisations need staff equipped with improvement skills to help them identify problems, test new ideas and make continuous improvements in care quality. This includes embedding these skills more explicitly in medical curricula and professional development, an area where the UK has previously lagged behind the US, Canada, Australia and other European countries.
- Provider organisations need to be supported to develop the necessary data infrastructure and analytical expertise to measure, understand and improve the quality of the care they provide. This includes improving data collection and linkage, and making sure the health service has skilled analytical teams who are well supported to develop their practice. It also includes training and development for clinicians and managers so that they can ask better questions of their analytical teams.,
In conclusion, our report makes the case for much greater emphasis on the role and status of adopters within the spread process, both in terms of how interventions are codified and how programmes are designed. We believe that to some extent we need to ‘flip’ the traditional focus of spread programmes from the efforts and activities of the innovator towards understanding and supporting adoption. This could be accompanied by more recognition and rewards for the adoption of innovation, rather than giving innovators all the prizes.
Ultimately, reproducing complex health care interventions is not easy. But attempts to support diffusion will stand the greatest chance of success when they accurately reflect the nature of the task adopters face.
Notes and references
- Perla R, Reid A, Cohen S, Parry G. Health care reform and the trap of the “iron law” [weblog]. Health Affairs. 2015. Available from: http://healthaffairs.org/blog/2015/04/22/health-care-reform-and-the-trap-of-the-iron-law (accessed 9 August 2018).
- Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AS, Patchen Dellinger E, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. New England Journal of Medicine 2009;360:491–499.
- Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of surgical safety checklists in Ontario, Canada. New England Journal of Medicine. 2014;370:1029–1038.
- Rossi P. The iron law of evaluation and other metallic rules. In Miller JL, Lewis M. Research in Social Problems and Public Policy. 4th edition. Greenwich, Connecticut: Jai Press; 1987. pp. 3–20.
- Howarth E, Devers K, Moore G, O’Cathain A, Dixon-Woods M. Contextual issues and qualitative research. In Raine R, Fitzpatrick R, Barratt H, Bevan G, Black N, Boaden R et al. Challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Health Services and Delivery Research. 2016;4(16):105–120.
- Rogers EM. Diffusion of Innovations. 5th Edition. New York: Simon & Schulster; 2003.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: Systematic review and recommendations. The Milbank Quarterly. 2004;82(4):581–629.
- Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implementation Science. 2011;6:42.
- Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews? BMJ. 2008;336:1472.
- Hoffmann T, Erueti C, Glasziou P. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ. 2013;347:f3755.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:979–83.
- Hawe P. Lessons from complex interventions to improve health. Annual Review of Public Health. 2015;36:307–323.
- Bauman LJ, Stein RE, Ireys HT. Reinventing fidelity: The transfer of social technology among settings. American Journal of Community Psychology. 1991;19:619–639.
- Guise JM, Chang C, Butler M, Viswanathan M, Tugwell P. AHRQ series on complex intervention systematic reviews-paper 1: an introduction to a series of articles that provide guidance and tools for reviews of complex interventions. Journal of Clinical Epidemiology. 2017;90:6–10.
- Wells M, Williams B, Treweek S, Coyle J, Taylor J. Intervention description is not enough: evidence from an in-depth multiple case study on the untold role and impact of context in randomised controlled trials of seven complex interventions. Trials. 2012;13:95.
- Bate P, Robert G, Fulop N, Øvretveit J, Dixon-Woods M. Perspectives on context: A selection of essays considering the role of context in successful quality improvement. London: The Health Foundation; 2014.
- Batalden M, Batalden P, Margolis P, Seid M, Armstrong G, Opipari-Arrigan L, et al. Coproduction of healthcare service. BMJ Quality & Safety. 2016;25:509–517.
- Lanham HJ, Leykum LK, Taylor BS, McCannon CJ, Lindberg C, Lester RT. How complexity science can inform scale-up and spread in health care: understanding the role of self-organization in variation across local contexts. Social Science and Medicine. 2013;93:194–202.
- Rickles D, Hawe P, Shiell A. A simple guide to chaos and complexity. Journal of Epidemiology and Community Health. 2007;61:933–937.
- Damiani E, Donati A, Serafini G, Rinaldi L, Adrario E, Pelaia P, et al. Effect of performance improvement programs on compliance with sepsis bundles and mortality: A systematic review and meta-analysis of observational studies. PLoS ONE. 2015;10(5):e0125827.
- Tarrant C, O’Donnell B, Martin G, Bion J, Hunter A, Rooney KD. A complex endeavour: an ethnographic study of the implementation of the Sepsis Six clinical care bundle. Implementation Science. 2016;11:149.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage Publications; 1997.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Quality and Safety. 2015;24:10.
- Plsek PE, Greenhalgh T. Complexity science: The challenge of complexity in health care. BMJ, 2001;323(7313):625–628.
- The Evidence Centre. Evidence scan: Complex adaptive systems. London: The Health Foundation; 2010.
- Shiell A, Hawe P, Gold L. Complex interventions or complex systems? Implications for health economic evaluation. BMJ. 2008;336:1281–1283.
- Hawe P, Shiell A, Riley T. Complex interventions: how “out of control” can a randomised controlled trial be? BMJ. 2004;328:1561–1563.
- Rogers PJ. Using programme theory to evaluate complicated and complex aspects of interventions. Evaluation. 2008;14(1):29–48.
- Rutter H, Savona N, Glonti K, Bibby J, Cummins C, Shiell A, et al. The need for a complex systems model of evidence for public health. The Lancet. 2017;390(10112):2602–2604.
- Tadd W. Surviving Sepsis: Formative evaluation of the implementation process. Northumbria Healthcare NHS Trust; 2017. Unpublished.
- Laverty A. Global problem, local action: tackling sepsis in Northumbria [weblog]. London: The Health Foundation; 2017. Available from: www.health.org.uk/blog/global-problem-local-action-tackling-sepsis-northumbria (accessed 9 August 2018).
- Roberts T. Northumbria Healthcare NHS Trust: Sepsis Six outcomes analysis. North East Quality Observatory Service; 2017. Unpublished.
- Dixon-Woods M, Bosk CL, Avelin EL, Goeschel CA, Pronovost PJ. Explaining Michigan: developing an ex post theory of a quality improvement program. The Milbank Quarterly. 2011;89(2):167–205.
- Dixon-Woods M, Graham M, Tarrant C, Bion J, Goeschel C, Pronovost P et al. Safer Clinical Systems: evaluation findings. London: The Health Foundation; 2014.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
- Draycott T, Sibanda T, Owen L, Akande V., Winter C, Reading S et al. Does training in obstetric emergencies improve neonatal outcome? British Journal of Obstetrics and Gynaecology. 2006;113:177–82.
- Shoushtarian M, Barnett M, McMahon F, Ferris J. Impact of introducing practical obstetric multi-professional training (PROMPT) into Maternity Units in Victoria, Australia. British Journal of Obstetrics and Gynaecology. 2014;121:1710–1718.
- Draycott T. Can understanding context improve how we deliver training? [webpage]. London: The Health Foundation; 2014. Available from: www.health.org.uk/newsletter/can-understanding-context-improve-how-we-deliver-training (accessed 9 August 2018)
- The Health Foundation. The PROMPT system: an innovation in peri-operative care [webpage]. London: The Health Foundation; 2018. Available from: www.health.org.uk/programmes/innovating-improvement/projects/prompt-system-innovation-peri-operative-care (accessed 9 August 2018)
- Dixon-Woods M. Final report on the evaluation of Safer Clinical Systems: findings from the extension phase. London: The Health Foundation; 2016. Unpublished.
- Microsystem Coaching Academy (MCA). Flow Coaching Academy (FCA) [website]. Available from: www.sheffieldmca.org.uk/flow/ (accessed 9 August 2018).
- Spring Impact. Scale Accelerator Insight 2016–2017 [website]. Available from: www.springimpact.org/2017/12/scaling-social-impact-uk-insights-scale-accelerator-2/ (accessed 10 August 2018).
- Coulter A. Implementing shared decision making in the UK: a report for The Health Foundation. London: The Health Foundation; 2009.
- Glidewell L, Boocock S, Pine K, Campbell R, Hackett J, Gill S, et al. Using behavioural theories to optimise shared haemodialysis care: a qualitative intervention development study of patient and professional experience. Implementation Science. 2013;8:118.
- Interview with programme evaluator.
- Interview with programme leader.
- Albury D, Beresford T, Dew S, Horton T, Illingworth J, Langford K. Against the odds: Successfully scaling innovation in the NHS. London: Innovation Unit; 2018.
- Perrigot R. Email to authors. 7 September 2017.
- Aveling EL, Martin G, Jiménez García S, Martin L, Herbert G, Armstrong N, et al. Reciprocal peer review for quality improvement: an ethnographic case study of the Improving Lung Cancer Outcomes Project. BMJ Quality & Safety. 2012;21:1034–1041.
- Whitehouse C, Dixon-Woods M. Using clinical communities to improve quality: Ten lessons for getting the clinical communities approach. London: The Health Foundation; 2013.
- Ling T, Stewart K, Garrod B, Hocking L, Newbould J, Gendronneau C. Evaluation of the second phase of the Q Initiative 2016–2020: Interim report. RAND Europe; 2018. Unpublished.
- Miller RL, Shinn M. Learning from communities: overcoming difficulties in dissemination of prevention and promotion efforts. American Journal of Community Psychology. 2005. 35(3/4):169–183.
- British Lung Foundation. Chronic Obstructive Pulmonary Disease (COPD) statistics [website]. London: British Lung Foundation; 2007. Available from: https://statistics.blf.org.uk/copd (accessed 9 August 2018).
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. European Respiratory Review. 2014;23(133):345–349.
- The Health Foundation. Promoting a positive life experience for COPD patients [webpage]. London: The Health Foundation; 2015. Available from: www.health.org.uk/programmes/shine-2014/projects/promoting-positive-life-experience-copd-patients (accessed 9 August 2018).
- University Hospitals Coventry and Warwickshire NHS Trust, Coventry University. Shine 2014 final report. RIPPLE: Respiratory Innovation: Promoting positive life experience. London: The Health Foundation; 2015.
- Dodd P. Spreading Improvement programme expression of interest: Making waves – promoting a positive life experience for COPD patients: Ripple 2. South East Staffordshire and Seisdon Peninsula Clinical Commissioning Group; 2014. Unpublished.
- Taylor A, Sewell L. Evaluation of the Making Waves: RIPPLE 2 Spread Project. Coventry University; 2018. Unpublished.
- Interview with implementation site lead.
- Interview with implementation site lead.
- Dodd P. Making Waves – Promoting a positive experience for people with COPD [conference presentation]. ISQua 2017 Conference. Programme available from: www.health.org.uk/sites/health/files/ISQua%20conference%20Programme%202017%20-%20draft.pdf (accessed 9 August 2018).
- Interview with innovator.
- Dixon-Woods M, McNicol S, Martin G. Evidence: Overcoming challenges to improving quality. London: The Health Foundation; 2012.
- Sutton E, Dixon-Woods M, Tarrant C. Ethnographic process evaluation of a quality improvement project to improve transitions of care for older people. BMJ Open. 2016;6:e010988.
- Lloyd T, Wolters A, Steventon A. Briefing: The impact of providing enhanced support for care home residents in Rushcliffe. London: The Health Foundation; 2017.
- Burd H, Hallsworth M. Spreading change: A guide to enabling the spread of person- and community-centred approaches for health and wellbeing. London: The Health Foundation; 2016.
- Briñol P, Petty RE. Source factors in persuasion: a self-validation approach. European Review of Social Psychology. 2009;20(1):49–96.
- Davies HTO, Nutley SM, Mannion R. Organisational culture and quality of health care. BMJ Quality & Safety. 2000;9:111–119.
- Stokes K, Barker R, Piggot R. Which doctors take up promising ideas? New insights from open data. London: Nesta; 2014.
- de Silva D (The Evidence Centre). Evidence scan No. 21: Improvement collaboratives in health care. London: The Health Foundation; 2014.
- Norton MI, Mochon D, Ariely D. The IKEA effect: when labor leads to love. Journal of Consumer Psychology. 2012;22(3):453–60.
- Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining Matching Michigan: an ethnographic study of a patient safety program. Implementation Science. 2013;8:70.
- Starling A. Some assembly required: implementing new models of care. London: The Health Foundation; 2017.
- National Audit Office. Developing new care models through NHS vanguards. London: National Audit Office; 2018.
- Wolfe I, Cass H, Thompson MJ, Craft A, Peile E, Wiegersma PA, et al. Improving child health services in the UK: insights from Europe and their implications for the NHS reforms. BMJ. 2011;342:1277.
- Sidebotham P, Fraser J, Fleming P, Ward-Platt M, Hain R. Patterns of child death in England and Wales. The Lancet 2014;384:904–914.
- Goldenhar L, Brady P, Sutcliffe K, Muething SE. Huddling for high reliability and situation awareness. BMJ Quality & Safety. 2013;22:899–906.
- Reece A. Safe prescribing tools: DRUG-gle (Druggle) [webpage]. Meds IQ. 2015. Available from: www.medsiq.org/tool/drug-gle-druggle (accessed 9 August 2018)
- Information provided by site lead.
- Evidence Based Practice Unit, University College London. S.A.F.E: Situation Awareness for Everyone: Health Foundation final report. London: The Health Foundation; 2016. Unpublished.
- Interviews with programme and implementation site leads.
- Stapley E, Sharples E, Lachman P, Lakhanpaul M, Wolpert M, Deighton J. Factors to consider in the introduction of huddles on clinical wards: perceptions of staff on the SAFE programme. International Journal for Quality in Health Care. 2018;30(1):44–49.
- Edbrooke-Childs J, Hayes J, Sharples E, Gondek D, Stapley E, Sevdalis N, et al. Development of the Huddle Observation Tool for structured case management discussions to improve situation awareness on inpatient clinical wards. BMJ Quality & Safety. 2018;27:365–372.
- Deighton J, Edbrooke-Childs J, Stapley E, Sevdalis N, Hayes J, Gondek D et al. Realistic evaluation of Situation Awareness for Everyone (SAFE) on paediatric wards: study protocol. BMJ Open. 2016;6:e014014.
- Royal College of Paediatrics and Child Health. S.A.F.E. resource 5 – Huddling [RCPCH website article]. 2018. Available from: www.rcpch.ac.uk/resources/safe-resource-5-huddling (accessed 9 August 2018).
- Jones B, Woodhead T. Building the foundations for improvement: How five UK trusts built quality improvement capability at scale within their organisations. London: The Health Foundation; 2015.
- Dorgan S, Layton D, Bloom N, Homkes R, Sadun R, Van Reenen J. Management in Healthcare: Why good practice really matters. London: McKinsey & Company; 2010.
- Jones L, Pomeroy L, Robert G, Burnett S, Anderson JE, Fulop NJ. How do hospital boards govern for quality improvement? A mixed methods study of 15 organisations in England. BMJ Quality & Safety. 2017:26:978–986.
- Academy of Medical Royal Colleges. Quality Improvement – training for better outcomes. London: Academy of Medical Royal Colleges; 2016.
- The Evidence Centre. Evidence scan: Quality improvement training for healthcare professionals. London: The Health Foundation; 2012.
- Deeny SR, Steventon A. Making sense of the shadows: priorities for creating a learning healthcare system based on routinely collected data. BMJ Quality & Safety. 2015;24:505–515.
- Bardsley M. Understanding analytical capability in health care: Do we have more data than insight? London: The Health Foundation; 2016.
Appendix: Projects from the Health Foundation’s spread-related funding programmes
* This project was also part of the Closing the Gap through Changing Relationships programme (2010–2013).
About the authors
Tim Horton is Assistant Director of Improvement at the Health Foundation.
John Illingworth was Improvement Fellow at the Health Foundation from 2012–2018 and was involved in the production of this report until March 2018.
Will Warburton is Director of Improvement at the Health Foundation.
This research originally appeared in a different version published by Project HOPE/Health Affairs as: Tim J. Horton, John H. Illingworth, and Will H. P. Warburton: “Overcoming Challenges In Codifying And Replicating Complex Health Care Interventions.” Health Affairs (Millwood) 2018, Vol. 37, No. 2, 191–197. The original published article is archived and available online at www.healthaffairs.org, https://doi.org/10.1377/hlthaff.2017.1161.